The reaction 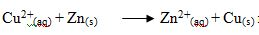 represents which among the following reactions.
represents which among the following reactions.
- Synthesis
- Precipitation
- Neutralization
- Displacement
- Decomposition
2
(vi) What type of chemical reaction is represented by the equation
Zn(s) + 2HCl(aq) + H2(g)?
A Displacement reaction
B Combination reaction
C Precipitation reaction
D Decomposition reaction
E Redox reaction
Choose Answer :3
(iii) A rapid chemical reaction that releases energy in form of light and heat is called
- combustion.
- decomposition.
- displacement.
- neutralization.
- precipitation.
4
Complete the following equations and determine the type of chemical reaction involved in each case.
(i) Zn(s) + H2SO4( aq) ?
(ii) AgNO3( aq) + NaCl( aq) ?
(iii) N2(g) + H2(g) ?
View Ans5
Give the name of the type of reaction represented by each of the following chemical equations.
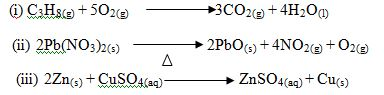
6
State and describe the type of reaction in the following chemical equations:
(i) Fe(s) + CuSO4(aq) à FeSO4(aq) + Cu(s).
(ii) Na2SO4(aq) + BaCl2(aq) à BaSO4(s) + 2NaCl(aq).
View Ans7
7. (a) Briefly explain five importance of balancing chemical equations.
View Ans8
(b) Give a balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid. (7 marks)
View Ans9
Classify the following reactions into oxidation and reduction reactions.
(i) S( s) + O 2( g) ? SO 2( g)
(ii) N2( g) + 3H 2( g) ? 2NH 3( g)
(iii) Fe2+ (aq) e ? Fe3+ (aq)
(iv) Fe3+ (aq) e ? Fe2+ (aq) (4 marks)
View AnsHub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






