A solution of pH 1.6 is best described as
- weak acid
- strong base
- weak base
- strong acid
- neutral solution.
2
As water is added to an acid, the acid becomes
- more acidic and its pH goes down
- more acidic and its pH goes up
- less acidic and its pH goes up
- less acidic and its pH goes down
- neutral and its pH becomes 7.
3
Hygroscopic and deliquescent substances can be used as
- oxidising agents
- drying agents
- reducing agent
- weak electrolytes
- catalyst.
4
Which of the following pairs of compounds can be used in the preparation of calcium sulphate?
- Calcium carbonate and sodium sulphate
- Calcium chloride and ammonium sulphate
- Calcium hydroxide and barium sulphate
- Calcium nitrate and lead (II) sulphate
- Calcium chloride and barium sulphate.
5
Which action should be taken immediately after concentrated sulphuric acid spilled on the skin?
- Its should be rinsed off with large quantities of running water.
- It should be neutralized with solid CaCO3
- It should be neutralized with concentrated NaOH.
- The affected area should be wrapped tightly and shown to a medical health provider.
- It should be neutralized with concentrated KOH.
6
The process of giving away water of crystallization to the atmosphere by a chemical substance is called
- efflorescence
- deliquescence
- hygroscopic
- sublimation
- vapourisation.
7
7. Classify the following salts on the basis of solubility in water: Sodium carbonate, Lead nitrate, Silver chloride, Copper (Il) sulphate, Barium sulphate, Zinc chloride and Lead sulphate.
View Ans8
Distinguish normal salts from acidic salts based on how they are formed.
View Ans9
Give four uses of salts in daily life.
View Ans10
With the aid of a chemical equation, describe how you would prepare pure solid sodium chloride by the action of an acid and a base.
View Ans11
Using four examples, explain how the process of neutralization is important in day to day life.
View Ans12
Differentiate dilute hydrochloric acid from dilute sulphuric acid.
View Ans13
A student tested five solutions M, N, O, P and Q with a universal indicator solution to find their pH values. The following results were obtained.
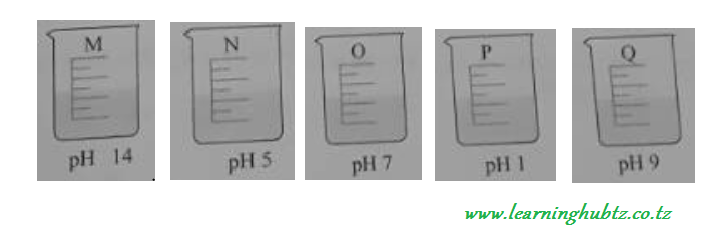
Which of the above solutions was?
-
Neutral solution
-
Strong acid
-
Strong alkali
-
Weak acid.
14
State the meaning of the following and give one example in each case.
-
Amphotenic oxide
-
Acidic oxide.
15
(i) People suffering from heart burn usually use wood ashes for relief. Mention characteristic which makes the ashes to be used for heart burn relief.
(ii)Give four compounds found in laboratories which show the same characteristics as ashes.
View Ans16
(i) Name the products formed when nitrates of potassium and zinc decompose by heat.
(ii) Suggest why the nitrates of zinc and potassium behave differently on heating.
View Ans17
Mention two uses of sodium nitrate.
View Ans18
SECTION B ( 70 Marks)
![]() Answer all questions in this section.
Answer all questions in this section.
3. (a) Different salts behave differently when heated. Use balanced chemical equations to show how carbonates and sulphates behave when subjected to heat.
(b) Ammonium nitrate does not react like other nitrates (with exception of the alkali metal nitrates). Explain this fact with the aid of chemical equations. (7 marks)
View AnsHub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






