Which of the following compounds does NOT belong to the alkenes homologous series?
- C2H4
- C3H6
- C4H 8
- C5H10
- C6H 14.
2
(vi) Which one is the molecular formula for prop-I -ene?
- C3H5
- CH3CCH
- C3H4
- HCH2CCH
- CH3CHCH2
3
When methane undergoes substitutional reaction with excess chlorine, what is the final product?
- Chloromethane
- Dichloromethane
- Trichloromethane
- Tetrachloromethane
- Monochloromethane

4
Which of the following hydrocarbons does NOT belong to the same homologous series as the others?
- CH4 B
- C3H 8
- C4H10
- C6H 12
- C2H 12.
5
Alcohols react with carboxylic acids to form a group of organic compounds called
- alkynes
- aldehydes
- ethers
- esters
- alkanols.
6
The following equation is a propagation step in the chlorination of methane:
- Cl2
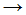 Cl• + Cl•
Cl• + Cl• - CH•3 + Cl•
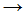 CH3Cl
CH3Cl - CH•3 + Cl2
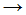 CH3Cl + Cl•
CH3Cl + Cl• - CH4 + Cl•
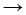 CH3Cl + H•
CH3Cl + H• - CH•3 + Cl2
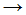 Ch2Cl + Cl•.
Ch2Cl + Cl•.
7
Which of the following equations represents the combustion of methane with the products collected at 120oC?
- CH4(l) +2O2(g) ?CO2(g) + 2H2O(l)
- CH4(g) +2O2(l) ?CO2(s) + 2H2O(l)
- CH4(g) +2O2(g) ?CO2(g) + 2H2O(g)
- CH4(l) +2O2(l) ?CO2(l) + 2H2O(g)
- CH4(l) +2O2(g) ?CO2(g) + 2H2O(g)
8
(iv)Which one is the molecular formula for prop-I-yne?
- C3H6
- CH3CCH
- C3H4
- HCH2CCH
- CH3CHCH2
9
The occurrence of two or more compounds with the same molecular formula but different molecular structures is known as.
- Amphoterism
- Isomerism
- Allotropy
- Polymorphism
- Isotopy
10
C2H4Cl can be represented in different structures which are called
- homologous series
- isomers
- structural formulae
- identical structures
- condensed structures.
11
Which of the following compounds does NOT belong to the alkane homologous series?
- C2H 4
- CH4
- C4H 10
- C3H 8
- C5H 12.
12
12. (a) Distinguish alkanes from alkenes by giving three points.
(b) Why carbon has been given special attention in organic chemistry rather than other elements? Give four reasons.
SECTION C (15 Marks)
Answer one (1) question in this section.
View Ans13
Define the following terms:
- Neutralization.
- Unsaturated solution.
- Thermal decomposition.
14
. (i) Define isomerism.
(ii) Draw and name two structural formulae of the isomers of C4H8.
View Ans15
Write the chemical formula of tetrachloromethane and state the type of bond that exists.
View Ans16
You are provided with CH3CH2OH, CH3CH2CH3, CH3COOH, and CH2 =CH2.
- Which compounds are gases at room temperature?
- How can you distinguish compound CH3CH2CH3 and CH2= CH2?
- Which compound would react with sodium carbonate? Write the balanced chemical equation for the reaction.
17
Write the structural formula for the following compounds:
-
But-2-ene.
-
Pent-2-yne
-
1,2-dichloroethane
-
2,4-dimethylhexane.
18
When a burning splint is introduced into a gas jar containinakes use of these two properties?
View Ans19
(a) (i) State three characteristics of a homologous series.
(ii) Draw the displayed/open structure formula of 2, 2-dichlorohexane.
(iii) Giving two reasons, explain why 2, 2-dichloro-3-methylbutane is a structural isomer of 2, 2-dichloropentane.
View Ans20
Write down the chemical equations of the reactions between the following:
(i) Ethanol and sodium metal.
(ii) Propanol warmed with excess acidified potassium permanganate.
(iii) Propanol and acetic acid warmed together in the presence of concentrated sulphuric acid.
View Ans21
Explain the following reactions giving one example in each:
(i) Addition reaction.
(ii)Elimination reaction.
View Ans
22
Consider a four carbon hydrocarbon (C4Hn), where n is an integer. Give the name of homologous series, molecular formula and structural formula for different isomers of the compound formed by each homologous. In each case indicate the causes of isomerism.
View Ans23
Which homologous series of organic compounds can be represented by the following general formula?
(i) Cn H2n+2
(ii) CnH2n
(iii) Cn H2 n+1OH (3 marks)
View Ans24
Give the name of the first compound in each series. (3 marks)
View Ans25
(a) Which homologous series of organic compounds can be represented by the following general formula?
(i) Cn H2n+2
(ii) CnH2n
(iii) Cn H2 n+1OH (3 marks)
(b) Give the name of the first compound in each series. (3 marks)
(c) (i) Describe a reaction by which a named compound of series in (a) (ii) can be converted to a compound of series in (a) (ii).
(ii) How can a compound of series (a) (iii) be converted to a compound of series in (a) (ii)? (4 marks)
View AnsHub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






