



FORM FOUR CHEMISTRY EXAM SERIES 217
FORM FOUR CHEMISTRY EXAM SERIES 217
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
PRE- MOCK CHEMISTRY FORM FOUR
Time: 3Hours
Instructions
- This paper consists of sections A, B, and C with a total eleven (11) questions.
- Answer all question in the sections A, B and two (2) questions from section C.
- Section A carries sixteen (16) marks, section B fifty four (54) marks and section C carries thirty (30) marks.
- All writing should be in blue or black pen, except for diagrams that must be drawn in pencil.
- Communication devices and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet (s)
SECTION A.
- For each of the following items (i-x).Choose the correct answer from given alternatives and writer its letter besides the item number in the answer booklet
- Which type of bonding is expected in a compound formed between potassium (K) and chlorine (Cl)?
- Ionic
- Covalent
- Metallic
- Hydrogen Bonding
- Dipole-Dipole Interactions
- The mass number of an atom represents the total number of:
- Protons only
- Neutrons only
- Electrons only
- Protons and neutrons
- Protons and electrons
- How many moles of carbon dioxide (CO2) are present in 88 grams of CO2?
- 0.5 moles
- 1 mole
- 2 moles
- 4 moles
- 88 moles
- When heating a flammable liquid in a laboratory, which of the following is the safest practice?
- Direct heating with a Bunsen burner
- Heating in a water bath
- Heating in an open test tube
- Adding flammable liquids to an open flame
- Leaving the experiment unattended
- Which separation technique would be most appropriate for separating a mixture of water and ethanol?
- Filtration
- Chromatography
- Simple distillation
- Fractional distillation
- Evaporation
- During the electrolysis of molten sodium chloride (NaCl), which of the following substances is formed at the cathode?
- Chlorine gas (Cl2)
- Sodium metal (Na)
- Hydrogen gas (H2)
- Oxygen gas (O2)
- Hydrochloric acid (HCl)
- For a reversible reaction, a large value of the equilibrium constant (Kc) indicates that:
- The reaction proceeds very slowly
- The forward reaction is favored
- The reverse reaction is favored
- The reaction is at equilibrium
- The reaction does not take place
- Which metal is commonly extracted from its oxide ore using electrolysis?
- Iron (Fe)
- Gold (Au)
- Sodium (Na)
- Copper (Cu)
- Zinc (Zn)
- Chlorine reacts with sodium hydroxide (NaOH) to form a common household disinfectant. What is the main active ingredient in this disinfectant?
- Hydrochloric acid (HCl)
- Sodium chlorate (NaClO3)
- Sodium hypochlorite (NaClO)
- Sodium chloride (NaCl)
- Sodium chlorite (NaClO2)
- Which type of reaction is most characteristic of saturated hydrocarbons?
- Substitution
- Addition
- Polymerization
- Condensation
- Combustion
2. Match the compound in LIST A with the correct way to identify it in LIST B and write the letter of the correct answer on sheet provided.
| LIST A | LIST B |
|
|
SECTION B: 54 Marks
3(a) State two reasons why we use the non-luminous flame for heating in a laboratory instead of using the luminous flame.
(b) Chlorine has two isotopes with atomic mass 35 and X occurring in the ratio 3:1 respectively. The relative atomic (R.M.A) of chlorine is 35.5. Determine the value of X.
(c) In an experiment to electroplate iron with silver, current of 1 Ampere was passed through a silver solution of ions for 60 minutes.
(i) Give a reason why it is necessary to electroplate iron.
(ii) Calculate the mass of silver deposited on iron during the electroplating process. (Ag = 108, IF = 96500c)
4. (a) Calculate the volume of 0.6M sulphuric (VI) acid solution needed to neutralize 30cm3 of 0.2Mpotassium hydroxide.
(b) A state of equilibrium between dichromate (vi) and chromate ions is established as shown below
Cr?O?²? (aq) + H?O (l) ? 2 CrO?²? (aq) + 2 H? (aq)
Orange (Yellow)
i. What is meant by dynamic equilibrium?
ii. State and explain observation made, when a few pellets of Potassium Hydroxide are added to equilibrium mixture
(c ) The following reaction takes place in a closed system:
N?(g) + 3H?(g) ? 2NH?(g) ΔH = -92 kJ/mol
Consider the following scenarios:
Scenario 1: More nitrogen (N?) gas is added to the system.
Scenario 2: The temperature of the system is decreased.
Scenario 3 A catalysts is added to the system.
For each scenario:
a) Predict the direction in which the equilibrium will shift (left, right, or no change). b) Explain your reasoning using the principles of chemical equilibrium and Le Chatelier's principle.
5. Use the diagram below to answer the questions that follow.
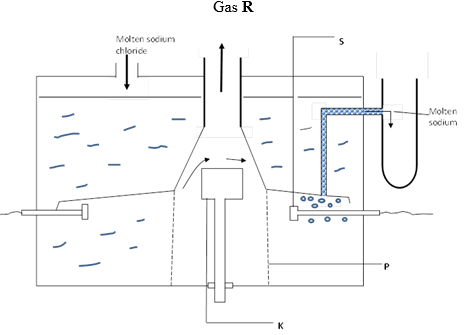
(a) Identify the substances labelled R, S, K and P (b) What is the function of the part labelled P?
(c) Write half equations at the electrodes.
(d) Why is molten sodium chloride used instead of sodium chloride solution?
(e) Why is calcium chloride added in the electrolysis of molten sodium chloride?
(f) How is the calcium eventually separated from the sodium?
(g) When sodium is left exposed in the air a white solid is formed but when sodium is burnt in oxygen, a yellow solid is formed. Explain this difference using equations.
6. Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
| Element | Atomic number | Melting point 0C |
| R | 11 | 97.8 |
| S | 12 | 650.0 |
| T | 15 | 44.0 |
| U | 17 | -102.0 |
| V | 18 | -189.0 |
| W | 19 | 64.0 |
(a) Give a reason why the melting point of;
(i) S is higher than that of R.
(ii) V is lower than that of U. (b) How does the reactivity of W with chlorine compare with that of R with chlorine?
(c) When 0.30g or R was reacted with water 1600cm3 of gas was produced. Determine the relative atomic mass of R. (Molar gas volume = 24000cm3 r.t.p
(d) Give one use of element V.
(e) Draw a structure of the compound formed when S reacts with U.
(f) Compare the atomic radius of element S and V. Give a reason
7. (a) Hard water has both advantages and disadvantages. Give one advantage and one disadvantage of using hard water
(b) Using an equation, explain how addition of sodium carbonate is used to remove water hardness.
(c) Outline three importance of a chemical equation.
8. I. In an experiment, copper metal was heated in the air to form a black solid T. dilute Sulphuric (VI) acid was then added to solid T resulting to formation of solution W, after which Ammonia was then added to solution W drop wise till excess
(a) Identify solid T
(b)Write a chemical equation for the reaction leading to formation of solution W
(c) State the observations made when the ammonia solution was added to solution W dropwise till excess.
II. Substance A is a solid that does not conduct electricity at room temperature. However, when molten, it becomes a good electrical conductor.
Substance B is a solid with a high melting point and can conduct electricity in the solid state.
a) Suggest the likely types of bonding present in Substance A and Substance B.
b) Explain the differences in their electrical conductivity in both solid and molten/liquid states.
(c) An experiment is set up to electrolyze a concentrated solution of sodium bromide (NaBr).
i) Identify the products that would form at the cathode and anode.
ii) Explain your reasoning, including relevant half-equations.
iii) Describe any observable changes expected during the electrolysis process.
SECTION B: 30 MARKS
Answer any two questions
9.(a) What name is given to each of the following?
(i) Ability of a metal to be beaten/ hammered to a sheet
(ii)Force of attraction that holds two molecules together
(b) When 3.1g of Copper {II} Carbonate were heated in a crucible until no further change in mass, solid L and gas M were formed
(i) Identify solid L and gas M
(ii) Write a chemical equation for the reaction that occurred
(iii) Given Cu=64, C=12,O=16, calculate the mass of the solid L that was formed
10 (a) Give the name of the following processes.
(i) A hot saturated solution of copper (II) sulphate is cooled to form crystals of copper (II) sulphate.
(ii)A white powder is formed when concentrated sulphuric (V) acid is added to blue hydrated copper
(II) sulphate.
(b)Study the flow chart below and answer the questions that follow.
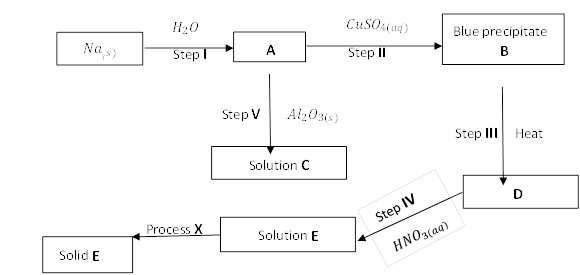
(i) Name substances: B, C, D, and Solid E
(ii) Write equations for the reactions in steps; III and V
(iii) Write the ionic equation for the reaction in step II.
(iv) State any two observations made in step I.
11. (a) Addition of inorganic fertilizers in the farm is not as important as addition of organic manure. Discuss the correctness of this statement in four (4) points
(b) Soil fertility and soil productivity are mistakenly used to mean the same concept. How do they differ from each other? Give five points
FORM FOUR CHEMISTRY EXAM SERIES 185
FORM FOUR CHEMISTRY EXAM SERIES 185
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM FOUR
TERMINAL EXAMS MAY – 2023
032/1
CHEMISTRY
TIME: 3HOURS
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of eleven (11) questions.
- Answer all questions in sections A and B and two (2) questions from section C.
- Non-programmable calculators may be allowed.
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer sheet(s)
- The following constants may be used: Atomic masses:
Atomic masses; H=1, O=16, Na=23, C=12, Cl=35.5, N=14, Zn=65, S=32,
Ca=40, Fe=56, Cu=64, Al=27
GMV at s.t.p = 22.4dm3, Avogadro’s number = 6.02 x 1023
1Faraday = 96500 coulomb 1 litre = 1dm3=1000cm3
SECTION (16 MARKS)
- For each of the items (i – x) choose the correct from the given alternatives and write it’s letter in the answer sheet provided
- A substance which absorb water from the atmosphere and form a solution is called ....
- Efflorescent
- Hygroscopic
- Deliquescent
- Amphoteric
- Technicians prefer to use blue flame in welding because
- It is bright and non soot
- It is light and non soot
- It is very hot and non soot
- It is not expensive
- Chlorine ion Cl- differ from chlorine atom because it has
- More proton
- Less proton
- More electron
- Less electrons
This is a good example of
- Neutralization reaction
- Double decomposition reaction
- Redox reaction
- Synthesis reaction
- The volume of 0.2M of H2SO4 acid required to neutralize completely 25.00cm3 of 0.05MKOH is...........
- 0.626cm3
- 3.125cm3
- 12.500cm3
- 6.315cm3
- An electric current was passed through concentrated hydrochloric acid using carbon electrodes. The substance liberated at the anode was
- Copper
- Chlorine
- Oxygen
- Hydrogen
- A good charcoal burns with................
- Luminous flame
- Non-luminous flame
- Very low energy value
- High production of gases
- A covalent bond is formed when
- Ammonia is formed
- Potassium and Oxygen combine
- A metal combines with nonmetal
- An atom loses an electron.
- The empirical formula of a certain compound is CH3. Its molar mass is 30g/mol. What will be its molecular formula?
- C2H6
- CH4
- C2H8
- C2H4
- ........ is the general terms used to explain a mixture of different metals
- Amphoteric
- Allotrope
- Isotope
- Alloy
- Match the item in LIST A with the correct response in LIST B
| LIST A | LIST B |
|
|
SECTION B (54 MARKS)
Answer all questions from this section
- (a)Write balanced equation of
- Sodium hydroxide react with Sulphuric acid
- Calcium carbonate decomposed by heat
(b)(i)Wit aid of a balanced chemical equation name the products formed when nitrates of potassium and zinc decompose by heat
(ii)Suggest why nitrates of zinc and potassium behave differently on heating
- (a)(i)People suffering from heart burn usually use wood ashes for relief.
Mention characteristics which make the ashes to be used for heart burn relief
(ii)Give four compounds founds in the laboratory which show the same characteristic as ashes.
(b)How many ions are there in 6.82g of Al2(SO4)3
- A student tested four sample of water each 10cm3 from different areas at Malawi village by shaking by shaking with tree drops of soap solution. The experiment was repeated a second time by boiling each sample of water (10cm3) with three drops of soap solution. The observations were recorded as shown in the table below.
| Sample | Observation with soap solution | Observation for boiled sample with soap |
| | No lather | Lather |
| | Lather | Lather |
| | Lather | Lather |
| | No lather | No lather |
- Which sample contains only temporary hard water? Give reason
- Which sample contains permanent hard water? Give reason
- Name the chemical substances that would be the causes of hardness in sample A
- Write the chemical equation for removing hardness in
- Sample A
- Sample D
- 5.3g of X2CO3 was dissolved in water to make 0.5 litres of a solution. 25cm3 of this solution required 50.0cm3 of 0.1MHCL for complete neutralization.
- Write the balanced chemical equation for the reaction
- Calculate the concentration of X2CO3 in mol/dm3
- Calculate the relative molecular mass of X2CO3
- Calculate the relative atomic mass of X
- What is the name and symbol of element X
- (a)Give two example in each of the following
- Solid fuel
- Gaseous fuel
(b)The reaction which produces methanol from carbon monoxide and hydrogen is represented by the equation
The reaction is carried out at high pressure to give a better yield of methanol.
- Explain why increase in pressure gives a better yield of methanol
- The value of
is negative. What does this tell about the reaction
- With reason state whether a high temperature or low temperature will give a better yield of methanol
- Draw the well labeled diagram of laboratory preparation of Oxygen using the mixture of Potassium Chlorate and Manganese (iv) Oxide.
SECTION C (30 MARKS)
Answer only two (2) questions from this section,
- With the aid of the balanced chemical equations explain the processes that occur in the blast furnace during extraction of iron.
- (a)Briefly explain the following observations with the help of equations
- White anhydrous copper(II) sulphate changes its colour to blue when water is added
- Vigorous reaction takes place when a small piece of sodium is placed in water.
- Addition of zinc metal into a solution of copper (II) sulphate result into decolonization of the solution and deposition of a brown solid substance
(b)Define the following terms and give one example in each case
- Weak acid
- Acidic-salt
- Potassium permanganate reacted with an acid A to produce gas B.
Then gas B is passed through water and finaly to concentrated
Sulphuric acid.
- State;
- The name of compound A and gas B
- Two chemical tests of gas B
- The method which is used to collect gas B. Give reason
- Briefly explain as to why;
- Gas B is passed through water and concentrated sulphuric acid
- The preparation of gas B is always done in a fume chamber
- Write the balanced chemical equation for the reaction between Potassium permanganate and acid A
FORM FOUR CHEMISTRY EXAM SERIES 149
FORM FOUR CHEMISTRY EXAM SERIES 149
THE PRESIDENT�S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM FOUR-2022
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14�questions
- Answer all questions in section A and B�and ONE (1) question from section C.
- Section A and C carries 15 marks, while�section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro�s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3�= 1000cm3
�SECTION A ( 15 Marks)
Answer all questions in this section
1. For each of the items (i)-(x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- The percentage by mass of sodium in Na2 CO310H2O is:-
- 16.08%
- 18%
- 23%
- 40%
- 80%
- Which of the following lists contains renewable sources of energy?
- Biogas, solar power and charcoal
- Hydro electric power, coal and battery
- Biogas, solar power and firewood
- Charcoal, petrol and tidal waves
- Biogas, solar power and tidal waves
- Equal volumes of water form five different sources are boiled in a dish until no water is left. Which of the following will leave behind no deposits?.......................
- Spring water
- Rain water
- Lake water
- Pond water
- Tap water
- A saturated blue solution was electrolyzed using carbon electrodes. A reddish brown layer was formed on cathode and a reddish brown liquid at anode which of the following solutions was electrolyzed?
- Iron (III) chloride
- Copper (II) sulphate
- Iron (II) bromide
- Copper (II) bromide
- Iron (III) sulphate
- The chemical formula of carbon dioxide is CO2. The number 2 beside O means that there___________
- Are two molecules of carbon dioxide
- Is half an atom of oxygen for each molecule of carbon dioxide
- Are two atoms of oxygen for each molecule of carbon dioxide
- Are two atoms of carbon for each molecule of carbon dioxide
- Are two molecules of oxygen
- Below is a list of general formulae of homologous series of some organic compounds 1. C2H2n+1OH��� � � � �2. CnH2n+2� �3. CnH2n+1�COOH �4. CnH2n-2� .The correct order of the formulae is,
- Alkanoic acids, alkanes, alcohols and alkenes
- Alcohols, alkenes, alkanoic acids and alkanes
- Alcohols, alkanes, alkanoic acids and alkenes
- Alcohols, alkynes, alkanoik acids and alkenes
- Alkanes, alcohols, alkanoic acids and alkenes
�
- Your friend�s clothes catch fire. Which of the following is most effective in extinguishing the fire?
- Wet sand
- Wet blanket
- Carbon dioxide extinguisher
- Foam extinguisher
- None of the above
- Which of the following pair of particles has the same electronic structure?
- Sodium atom and chloride ion
- Carbon atom and chloride ion
- Calcium atom and chloride ion
- Chloride ion and neon atom
- Chloride atom and neon ion
- Exactly 50cm3 of a gas weighs 0.125g at S.T.P the relative molecular mass of the gas must be :-
- 56
- 44
- 22.4
- 100
- 32
- Which of the following fertilizers can be applied to neutralize the pH of slightly alkaline soil?
- Ammonium sulphate
- Urea
- Farmyard manure
- Compost manure �E.sisal wastes.
- Match the items in list A with the correct responses from list B
| List a� | List b |
|
|
�
�
�
�
�
�
SECTION B (70 marks)
Answer all questions in this section
- (a) (i) What observable changes occurs when hydrated iron (II) sulphate crystals are heated mildly and then strongly in a test tube?
(ii) Write two balanced equations for the above reactions
(b) When crystals of hydrated sodium carbonate (Na2CO3xH2O) where heated strongly only 37.06% by weight of the salt remained. How can you show that the value of x in Na2CO3 .xH2O is 10?
- I. �A Green solid T was heated until there was no further change. The following observations were made;
- Colourless liquid condensed on cooler parts of the test-tube
- A colourless gas which turns acidified potassium dichromate(VI) green was formed
- Red-brown residue S was formed
- Identify solid T
- Name the substance that could be used to identify colorless liquid
- Name the residue S
- Write equations for reactions that took place
- Why is iron not used to make steam boilers?
Study the flow chart below and answer questions that follows.
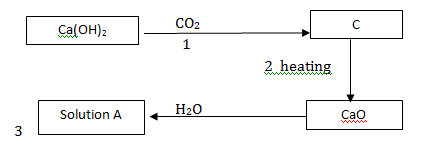
- What are the identities of solution A and solid C? Write chemical name and formulae
- What the day to day use of the conversion marked 2?
- (a) What is the role of a catalyst in a chemical reaction?
(b) Draw an energy level diagram for the decomposition of potassium chlorate when a catalyst: (i) is used (ii) not used
6. � (a) � �Briefly explain the concept of scientific procedure.
(b) � What is the importance of the scientific procedure in daily life? Give two points.(7 marks)
7.��Study the information in the table below and answer the questions that follow;
| Element� | Atomic number | Boiling point |
| A | 3 | 1603 |
| B | 13 | 2743 |
| C | 16 | 718 |
| D | 18 | 87 |
| E | 19 | 1047 |
�
- Select the elements which belongs to the same period and group
- Which element is
- Gaseous at room temperature
- Does not form an oxide, give a reason
- Write the formula of nitrate of B
�
8(a) Why is a fertile soil not necessarily productive?-give 2 points.
(b)(i) why is liming material added to soil?
� � �(ii) What are the causes that make the soil in need of liming material?-give 2 points.
9(a) Why do iron window frames rust quickly even though they are painted while aluminium� � �frames are resistant to rusting?
(b)Why are aluminium vessels used to transport nitric but not sulphuric acid?
10(a) (i) What is meant by fuel?
� � �(ii) How many classes of fuels are there?
(b)(i) The traditional process of making charcoal in an earth mould or pit kiln is wasteful process. Briefly explain.
� � ��ii)what is the effect of using charcoal on the environment?
11(a) (i) What is the relationship between athanoic acid and ethanol?
� � �(ii) How does the acidity of ethanoic differ to that of sulphuric acid?
(b)How does ethanoic acid react with:- (i)sodium metal? (ii) sodium carbonate solution?
SECTION C (15 MARKS)
Answer all question in this section.
12. During electrolysis one ion is discharged at each electrode. When two or more ion of similar charge are present in an electrolyte, only one ion is selected for discharge.
The selection depends on different factors. Explain how these factors affect the selection. Giving examples and relevant equations in each case.
13.You are in Amboni district in Tanga region where there is potential sulphur deposit. The district development society aims to extract the sulphur and has come to you for advice. Explain to the society.
- The equipment needed to the extraction. Use an illustration to make the explanation clear.
- The process involved during and other the extraction
- The effect of extraction on the environment
- Commercial uses of sulphur � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � ��
13.���(a)����Give three ways in which environmental destruction is likely to occur during extraction of metals.
(b)���The following equations represent the steps involved in the conversion stages of iron extraction in Bussener converter. Arrange the equations in chronological order from the first step to the last by writing the respective letter so as to get a complete explanation of the conversion stage.
�V: 2Cu20(S) +���Cu2S(S)?�����6Cu (l) + S02�(g)
�W:��FeO (l) + SiO2(g) ? FeSiO3
�X: 2Cus(s) +302�(g)�?����������2Cu20(s) +2S02�(g)
�Y: 2FeS (l) +302�(g) ? 2Feo(1) + 2S02�(g)���
�
1
�
14. Using a well labelled diagram, explain how sulphur is extracted using the Frasch process
FORM FOUR CHEMISTRY EXAM SERIES 88
FORM FOUR CHEMISTRY EXAM SERIES 88
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRYTERMINALEXAMINATION
FORM FOUR-2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) Which one of the following sets of laboratory apparatus are used for measure volume?
- Crucible, U-tube and volumetric flask
- Test tubes, beakers and glass jar
- Thistle funnel, separating funnel and beaker
- Burette, pipette and measuring cylinder
- Conical flask, test tube and measuring cylinder.
(ii) The empirical formula of certain compound in CH3. Its molar mass is 30 g. What will be its molecular formular?
- CH4
- C2H4
- C2H6
- C2H8
- C4H12
(iii) In order to produce the greatest amount of hydrogen in a short time, one gram of magnesium ribbon should react with
- 10 cm3 of 0.5 M sulphuric acid
- 40 cm3 of 0.5 M acetic acid solution
- 40 cm3 of 0.5 M sulphuric acid solution
- 20 cm3 of 1 M sulphuric acid solution
- 20 cm3 of 1 M acetic acid solution.
(iv) Fractional distillation process of a mixture of water and ethanol is possible because
- water and ethanol have the same boiling point
- water has lower boiling point than ethanol
- ethanol has lower boiling point than water
- water and ethanol form partially immiscible liquid solution
- water and ethanol are immiscible liquids.
(v) Which of the following substances represent a group of acidic oxides?
- Carbon dioxide, carbon monoxide and sulphur dioxide
- Sulphur trioxide, nitrogen dioxide and nnitrogen monoxide
- Carbon dioxide, sulphur dioxide and dinitrogen oxide
- Sulphur trioxide, carbon dioxide and nitrogen dioxide
- Carbon monoxide, nitrogen oxide and sulphur dioxide.
(vi) What will the molarity of a solution which contains 26.5 g of anhydrous sodium carbonate in 5 dm3 of solution?
- 0.05 M
- 0.25 M
- 5.30 M
- 0.025 M
- 0.50 M
(vii) The Brownian movement is taken to be the evidence of the:
- theory of association of water molecules
- theory of ionization of electrolytes
- theory of colloidal suspensions
- kinetic theory of behavior of substances
- Brownian theory.
(viii) One off the isotopes of an element X has an atomic number Z and a mass number A. What is the number of neutrons contained in the nucleus of the element X?
- Z
- A
- A + Z
- A – Z
- Z – A
(ix) C2H4Cl can be represented in different structures which are called
- homologous series
- isomers
- structural formulae
- identical structures
- condensed structures.
(x) _____ is the general term used to explain a mixture of different metals.
- Alloy
- Allotrope
- Amphoteric
- Amorphous
- Isotope.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3.A Form Three student conducted an experiment to prepare a gas in the laboratory by decomposing a certain compound using electricity. She allowed a steady electric current to flow through the solution for 3 hours at s.t.p. If the volume of the gas obtained was 4.12 dm3 and the gas relighted a glowing splint;
(a)name the gas that was produced.
(b)calculate the electric current that was flowing in the solution.
4. (a)An atom M has an atomic number 14 and mass number 28.
(i)What is the number of protons and neutrons?
(ii) Write the electronic configuration of atom M.
(b) Calculate the volume of water which was produced when 1,120 cm3 of oxygen at s.t.p. was liberated during the decomposition of hydrogen peroxide. The density of water = 1.0 g/cm3
5. An experiment was carried out to prepare crystals of magnesium sulphate.
Excess magnesium powder was added to l00cm'of dilute sulphuric(VI) acid in a beaker and warmed until no further reaction took place.
The mixture was filtered and the filtrate evaporated to saturation, then left to cool for crystals to form.
(a) (i) Write an equation for the reaction.
(ii) Explain why excess magnesium powder was used.
(iii) State how completion of the reaction was determined.
(iv) What is meant by a saturated solution?
(v) Explain why the filtrate was not evaporated to dryness.
6. (a) The diagram in Figure 4 was used to prepare hydrogen chloride gas which was passed over heated iron powder.
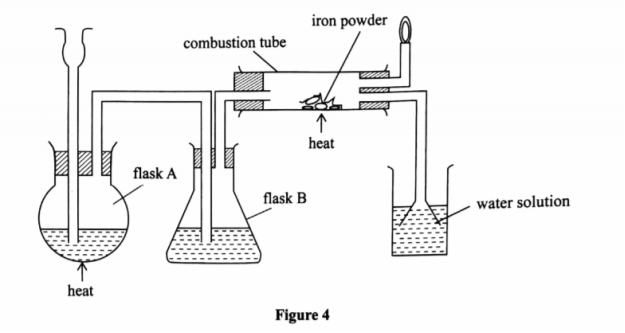
(i) Give a pair of reagents that will produce hydrogen chloride gas in flask A.
(ii) Name the substance in flask B.
(iii) State the observation made in the combustion tube.
(iv) Write an equation for the reaction in the combustion tube.
(v) Describe a chemical test for hydrogen chloride gas.
(b) (i) Identify the gas that bums at the jet.
(ii) Explain why the gas in (b) (i) is burned.
(b) Write an equation for the complete combustion of ethane.
(c) Give reasons why excess hydrogen chloride gas is dissolved using the funnel arrangement.
(d) State what will be observed when the reaction in the combustion tube is complete.
7. The set-up in Figure 2 was used to prepare a sample of ethane gas. Study it and answer the questions that follow.
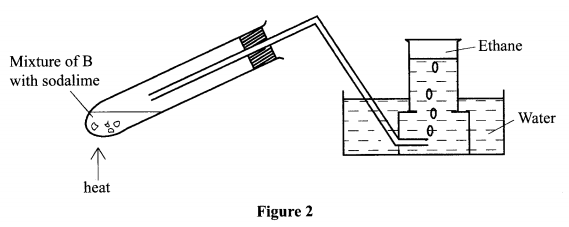
(a) Name B - sodium propanoate/:
(b) Write an equation for the complete combustion of ethane.
(c) State one use of ethane.
8. (a) A sample of water is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of sulphate ions.
(b) State one characteristic of a reaction where equilibrium has been attained.
9. Copper(II) ions react with excess aqueous ammonia to form a complex ion.
(a) (i) Write an equation for the reaction that forms the complex ion.
(ii) Name the complex ion.
(b) Explain why CH4is not acidic while HCl is acidic yet both compounds contain hydrogen.
10. The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
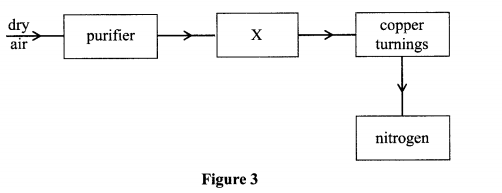
(a) Identify X (I mark)
(b) Write an equation for the reaction with heated copper turnings.
(c) Name an impurity in the sample of nitrogen gas.
11. Study the flow chart in Figure 5 and answer the questions that follow.
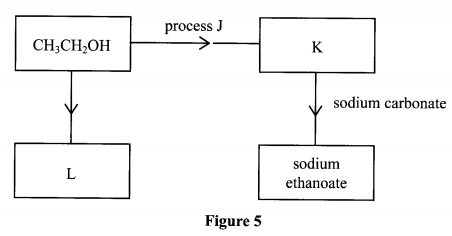
(a) Identify substances K and L. K:
(b) Name one reagent that can be used to carry out process J.
FORM FOUR CHEMISTRY EXAM SERIES 48
FORM FOUR CHEMISTRY EXAM SERIES 48
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- MOCK- EXAMINATION-MAY
FORM FOUR
Time 3:00 Hours MAY 2020
INSTRUCTIONS.
- This paper consist of three sections A, B and C
- Answer all questions in section A and B and one question in section C.
- The following constants may be used; H= 1. C=12. O= 16, Ag = 108, Cu =63.5
1 Litre = 1dm3 = 1000cm3
G.M.V at STP = 22.4dm3
Avogadro’s constant = 6.02 x 10 23 particles.
SECTION A: 15 MARKS.
- MULTIPLE CHOICE QUESTIONS.
(i) Which among the given list of metals arranged in order of decreasing reactivity with steam from left to right is correct?
- Calcium, Magnesium, Silver, Copper.
- Calcium, Magnesium, Zinc Copper
- Copper, Zinc, Magnesium, Calcium
- Calcium, Zinc, Magnesium Copper.
- Calcium, Magnesium, Zinc, Copper
(ii) A study current of 4A was passed through an aqueous solution of Copper Sulphate for 1800seconds. Mass of Copper deposited will be:
- 63.5
- 31.75g
- 1.185g
- 2.37g
- 11.8g.
(iii) Which of the following statement is true? Avogadro’s Constant is the number of?
- Element in one mole of solid substance
- Atoms in one mole of any gas at S.T.P
- Atoms in one mole of a metal
- Elements needed to liberate one gram of univalent metal
- Elements released when one mole of an element is discharged at the anode.
(iv) An organic compound of structural formula …………………………… belongs to homologous series of:
- Isomers
- Allotropes
- Molecules
- Radicals
- Isotopes.
(v) The atmosphere effect of burning fuel such as wood and petrol oils is to:
- Reduce Oxygen
- Produce clouds
- Add carbon dioxide
- Increase water vapour
- Produce energy.
(vi) Elements loose or gains electrons to form.
- Isotopes
- Radicals
- Molecules
- Ions
- Allotropes.
(vii) Which of the following groups of organic compounds is prepared by dehydration of corresponding alcohol?
- Alkynes
- Alkenes
- Alkanes
- Esters
- Carboxylic acid
(viii) Which of the following is most ductile?
- Alluminium
- Silver
- Copper
- Tin
- Mercury.
(ix) The same current passing through solution of the same concentration of silver nitrate and Copper Sulphate liberates 0.23g of Silver (equivalent weight = 108). The weight of Copper that will be liberated, (equivalent weight 31.8) is?
- 318g
- 0.0677g
- 0.23g
- 0.033g
- 3.18g
(x) In a blast furnace carbon monoxide is prepared by passing carbon dioxide over red – hot coke. Carbon dioxide is:
- An accelerator
- An Oxidizing agent
- A Reducing agent
- A catalyst
- Oxidized.
2. Match the items in List A with responses in List B by writing the letter of correct response beside the item number in List A.
| LIST A | LIST B |
| (i) Isomers (ii) Polymerization (iii) Ethanoic acid (iv) Lubricating oils (v) Homologous series
|
|
SECTION B:
3. (a) Give the reason for use of carbon dioxide
(i) As a fire extinguisher ( ½ marks)
(ii) As a refrigerant ( ½ marks)
(iii) In baking. ( ½ marks)
(b) Explain what will happen when carbon monoxide reacts with:
(i) Oxygen ( 1 marks)
(ii) Concentrated sodium hydroxide ( 1 marks)
(iii) Copper II Oxide. ( 1 marks)
(c) (i) Outline steps in preparation of charcoal ( 11/2 marks)
(ii) Mention two chemical properties of charcoal. (1 marks)
4. (a) Give two example for each or the following
(i) Strong acid ( 1 marks)
(ii) Strong alkali ( 1 marks)
(b) Identify the products formed when strong acid react with
(i) CuO(s) ( 11/2 marks)
(ii) NaOH(aq) ( 11/2 marks)
(c) Explain the meaning of the following and give two examples in each case.
(i) PH scale of an acid ( 1 marks)
(ii) Organic Acid. ( 1 marks)
5. (a) Describe the effect of:
(i) Strongly heating a piece of marble in Bunsen burner flame. ( 11/2 marks)
(ii) Moistening the residue (1) above with water. ( 11/2 marks)
(b)
(i) For what reason is slaked lime added to soil in gardening? ( 2 marks)
(ii) Why is concentrated sulphuric acid used as drying agent? ( 2 marks)
6. The preparation of ammonia M the laboratory is done by heating a mixture of ammonium chloride and sodium hydroxide.
(a)
(i) Write a balanced equation for the reaction ( 11/2 marks)
(ii) Use equations to show how ammonia reacts with hydrogen chloride gas and healed Copper II Oxide. ( 11/2 marks)
(b)
(i) State two uses of ammonia ( 1marks)
(ii) Name the catalyst used in preparation of ammonia ( 1marks)
(c) Explain each of the following reactions giving observation and equations.
(i) Aqueous ammonia is added to iron (III) Chloride, little by little until in excess. ( 1marks)
(ii) Sodium nitrate is strongly heated. ( 1 marks)
7.
a) Draw a well labeled diagram of non – luminous flame of Bunsen burner. 1 marks)
b) Explain the meaning of:
(i) Malleable ( 1/2 marks)
(ii) Ductile ( 1/2 marks)
(iii) Brittle ( 1 marks)
(c) Give an account for the following:
(i) Anhydrous Copper II Sulphate becomes coloured when exposed to air for long time.
( 1 marks)
(ii) Carbon dioxide can be collected by down ward delivery method. ( 1 marks)
(iii) Concentrated sulphuric acid is not used for drying hydrogen sulphide. ( 1 marks)
(iv) Sodium metal is kept in paraffin oil. ( 1 marks)
8.
a) Element A, B, C and D have atomic numbers 6, 8, 17 and 20 respectively. Write electronic structure of those elements ( 2 marks)
b) Write down the formulae of simplest compounds you would expect when;
(i) A and B combine chemically ( 1/2 marks)
(ii) C and D combine chemically ( 1/2 marks)
c)
(i) What types of bonding you would expect between compounds above? ( 1 marks)
(ii) List three differences between bonds you have identified above ( 3 marks)
9. (a)
(i) Name the product formed when nitrate of potassium and Zinc decompose by heating.
( 11/2 marks)
(ii) Suggest why the nitrate of Zinc and potassium behave differently when heating.
( 11/2 marks)
(b) Mention four uses of sodium nitrate. ( 4 marks)
10.
a) Giving four reasons, explain why people who use hard water can expect higher costs than people who use soft water. ( 3marks)
b) Suggest one method for separation of each of the following
(i) Iodine and sand ( 1marks)
(ii) Green solution form leaves ( 1marks)
(iii) Alcohol and water ( 1marks)
(iv) Iron fallings and powdered calcium carbonate. ( 1marks)
11. (a) A current of 0.5A were made to flow through silver voltmeter for 30 minutes. Calculate mass of silver deposited and equivalent weight of silver. ( 2marks)
(b) Explain the following reactions giving one example in each.
(i) Addition reaction ( 2marks)
(ii) Elimination reaction. ( 2marks)
12. (a) With an aid of chemical equations, explain the following terms,
(i) Esterification reaction ( 1marks)
(ii) Substitution reaction ( 1marks)
(iiii) Double decomposition reaction ( 1marks)
(b) Give a reason why alluminium is used in;
(i) Cooking Utensils ( 1marks)
(ii) Overhead Electricals ( 1marks)
(iii) Window Frames ( 1marks)
SECTION C:
Answer Only one Question.
12. Describe the two causes, two effects and measures to be undertaken in order to prevent/reduce the amount of acidic rain. (15 marks)
13. Consider the following.

Use Le Chaletires Principle to describe how the rate of production of D can be altered. (15 marks)
FORM FOUR CHEMISTRY EXAM SERIES 12
FORM FOUR CHEMISTRY EXAM SERIES 12
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







