THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY FORM TWO PRE- MOCK EXAMINATION
CODE 032
TIME: 2:30 HOURS
INSTRUCTIONS.
- This paper consists of section A, B and C with the total number of ten(10) questions
- Answer all questions in each section
- Section A carries (15) marks, section B (70) marks and section C carries (15) marks
- All writing must be in blue/black ink except drawing which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A (15 Marks)
Answer all questions in this section
1. For each of the items (i) – (x) choose the correct answer from among the given alternatives and write its letter beside the item number in the answer sheet provided
- Which of these is NOT a branch of chemistry?
- Organic Chemistry
- Biochemistry
- Astrology
- Physical Chemistry
- A student mixes two clear liquids and observes a bright yellow solid form and settle at the bottom. This is likely evidence of:
- Physical change
- Chemical change
- Evaporation
- No change occurring
- What's the first step if a chemical splashes into a student's eyes in the lab?
- Rub the eyes vigorously
- Get the teacher's attention
- Go directly to the nurse
- Flush eyes with water at an eyewash station
- Which of these is a compound?
- Copper (Cu)
- Saltwater
- Carbon Dioxide (CO2)
- Air
- The main component of the air we breathe is:
- Oxygen
- Carbon dioxide
- Nitrogen
- Helium
- What three conditions are needed for fire to burn?
- Fuel, water, air
- Fuel, oxygen, heat
- Heat, light, water
- Wind, oxygen, fuel
- A Bunsen burner's hottest flame is achieved when it is:
- Tall and yellow
- Roaring and blue
- Short and orange
- Flickering and red
- A glowing splint will relight when placed in a container of pure oxygen. This shows that oxygen:
- Supports combustion
- Creates water
- Is a product of combustion
- Is not flammable
- The characteristic "pop" sound in a hydrogen gas test indicates:
- Hydrogen forms water quickly
- Hydrogen is flammable
- Hydrogen is lighter than air
- Hydrogen is odorless
- Most of an atom's mass is concentrated in the:
- Nucleus
- Protons
- Electrons
- Electron shells
2. Match the items that tend to rust with the method for preventing it.
| Column A | Column B |
|
|
SECTION B: 70 MARKS
- The diagram below shows the relationship between the physical states of matter. Study it and answer the questions that follow.
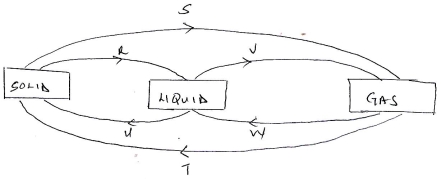
- Identify the process R,V,W and U
- Name three substances which can undergo the process represented by process S and T.
- (a) The table below shows liquids that are miscible and those that are immiscible
Liquid L3 L4
L1 Miscible Miscible
L2 Miscible immiscible
Use the information given to answer the questions that follow.
- Name the method that can be used to separate L1 and L3 from a mixture of the two.
- Draw and name an apparatus that can be used to separate a mixture of L2 and L4.
- Give two reasons why most Laboratory apparatus are made of glass.
- Name three sources of heat beside Bunsen burner in the laboratory.
- a) Draw a labeled diagram of a non-luminous flame produced by the Bunsen burner
b) State two reasons why a non-luminous flame is preferred for heating.
c) After use a non-luminous flame should be put off or adjusted to a luminous flame. Explain.
- (a) Name three apparatus that are used to measure accurate volume of liquids.
- Distinguish between an element and a compound and give an example of each.
- By use of a diagram between a residue and a filtrate.
- (a) Name the method you would use to separate the following mixtures.
- Sand and ammonium chloride.
- Oil and Water.
- Kerosene and crude oil
- Salt and water.
- Describe how you would separate a mixture of salt, sand and iodine into different components.
- (a)State the functions of the following apparatus as used in the laboratory.
- Spatula
- Pine-clay triangle
- Wire gauze
b) Draw and state the use of a deflagrating spoon.
(c )State the two causes of accidents in a Chemistry laboratory. (2mks)
- (a)Define the following terms
- Solvent extraction
- Hydrated salt
- Saturated Solution
- State two functions of a fume cupboard as found in a chemistry laboratory.
(c)Explain the differences between solid and gaseous states using the theoretical model of matter in terms of the Kinetic theory.
SECTION C: 15 Marks
10.
I. (a) Common table salt is contaminated with copper (ii) oxide. Explain how Pure sodium Chloride can be obtained from the mixture.
- The table below gives information on some substances. Use it to answer the question that follows.
| Substances | Melting Point oC | Boiling point oC | Solubility in water |
| A | -177 | 78.5 | Very soluble |
| B | -23 | 77 | insoluble |
| C | -219 | -183 | Slightly soluble |
| D | -78 | -33 | soluble |
- Which substance has the
- Lowest melting point
- Highest boiling point
- Which letters represents a substance that is a gas at room temperature?
- Which is a liquid at room temperature and when mixed with water two layers would be formed.
- Which substance dissolves in water and could be separated from the solution by fractional distillation.
- a) Give the symbols of the following elements
- Sodium
- Calcium
- Potassium
- Name the elements presents in the following compounds
- Zinc sulphide
- Sodium oxide.
FORM TWO CHEMISTRY EXAM SERIES 173
FORM TWO CHEMISTRY EXAM SERIES 173
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM TWO
TERMINAL EXAMS-MAY– 2023
033
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carry seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
- For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
(i) What is the best way of preparing hydrogen gas in the laboratory?
- By reacting strong metals and dilute acids.
- By reacting metals and acids.
- By reacting moderate metals and concentrated acids.
- By reacting moderate metals and dilute acids.
- By reacting strong metals and strong acids.
(ii)Consider the following reagents:
- H2O2
- H2O
- MnO4
- .MnO2
Which reagents are involved in the preparation of oxygen gas in the laboratory?
- I and 2
- 3 and 4
- CI and 3
- 2 and 3
- I and 4
(iii)A good fuel is the one which has
- high speed of continuous energy supply.
- high energy value supplied.
- low carbon dioxide supplied.
- high carbon dioxide production.
- high content of non-combustible material.
(iv) Which of the following pairs constitute the best methods for treating and purifying water?
- Chlorination and aeration
- Chlorination and decantation
- Chlorination and filtration
- Chlorination and sedimentation
- Chlorination and distillation
(v) A certain liquid dissolves copper (Il) sulphate to form a blue solution. This liquid is likely to be:
- Hydrochloric acid
- Liquid oxygen
- Nitric acid
- Water
- Lime water
(vi) Isotopes are atoms of the same element that have different:
- Atomic number
- Electron arrangement
- Mass number
- Protons
- Neutrons
(vii) An important property of oxygen which distinguishes it from other gases is that it:
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor supports combustion
- Supports combustion but does not burn
- Reacts with metals and Non-Metals
(viii) Which of the following electronic configurations are of metals?
A. 2:8:1 and 2:5
B. 2:8:2 and 2:6
C. 2:8:3 and 2:8:8:7
D. 2:8:6 and 2:8:8:7
E. 2:8 and 2:8:7
(ix) When a burning fuel produces blue colour it means there is:
- adequate supply of oxygen with production of soot
- inadequate supply of oxygen with production of more heat.
- inadequate supply of oxygen with production of soot.
- adequate supply of oxygen with production of more heat.
- Insufficient fuel
(x) The process of coating iron or steel with zinc is known as:
- zinc painting.
- alloying.
- tin plating.
- galvanization.
- Painting.
2. Match the description of a gas in list A with corresponding gas in List B and write the answer in the space provided.
| LIST A | LIST B |
|
|
SECTION B. 70 MARKS- ANSWER ALL QUESTIONS IN THIS SECTION
3. (a) Define the following terms as used in chemistry.
- Atom
- Atomic number
- Mass number
- Radicals
- Isotopes
(b) The table below shows the relative atomic masses and the percentage abundance of the isotopes L1 and L2 of element L.
|
| Relative atomic mass | % abundance |
| L1 | 62.93 | 69.09 |
| L2 | 64.93 | 30.91 |
Calculate the relative atomic mass of element L.
4.(a) The grid given below represents part of the periodic table. Study it and answer the questions that follow. (The letters do not represents the actual symbol of the elements)
- What name is given to the group of elements to which C and F belong?
- Compare the atomic radius of element C and F. explain
- Which letter represents the element that is the least reactive?
- What type of bond is formed when B and E react? Explain
- Write the formula of the compound formed when element D and oxygen gas react.
- On the grid, indicate with a tick the position of element G which is in the third period of the periodic table and forms G3- ions
(b) The table below gives the number of electrons, protons and neutrons in substances X, Y and Z.
Study it and answer the questions that follow.
| Substance | electrons | protons | neutrons |
| X | 10 | 10 | 10 |
| Y | 10 | 8 | 10 |
| Z | 8 | 8 | 8 |
(i) Which element represents and Ion
(ii) Which of the substances are isotopes? Give a reason.
5.(a) An organic compound P consist of 52.2% of carbon, 13% of hydrogen and 34.8% of oxygen. The vapour density of P is 23. Calculate the molecular formula of the compound P and write possible isomer(s) from the molecular formula determined.
(b) Hydrogen peroxide breaks down slowly to form water and oxygen; the reaction can be speed up by using a catalyst.
- How does the catalyst speed up the rate of reaction?
- Name a possible catalyst that can be used to speed up the reaction.
- Show that the catalyst always remains unchanged at the end of the reaction.
6.(a) Briefly explain the following
(i) Nitrogen gas is a necessary component of atmosphere
(ii) Nitrogen I Oxide is also called a Laughing gas
(iii) When carbon dioxide is passed through lime water, it turns milky
(iv) Things made of iron rust faster in coastal towns like Dar es salaam than in areas like Dodoma.
(v) Laboratory should have large windows
7.Using a well labelled diagram, explain how simple distillation can be used to separate a mixture of water and alcohol.
8. (a)What properties of hydrogen gas that made it to be used in the following?
i. As a fuel
ii. To fill weather balloons
iii. Manufacture of hydrochloric acid
iv. Manufacture of margarine
(b) Environmental pollution in most of rural areas in Tanzania is caused by using charcoal and firewood as a fuel. What will be the two alternative source of energy, they supposed to use for environmental conservation? (give reason for each)
(c) Why fossil fuels are referred to as non-renewable energy resources? (Give two reasons)
9. (a) (i) A table salt is a common name for the compound with the formula NaCl.
Write the systematic name for the table salt
(ii) Briefly describe why molecular formula better preferred than empirical formula is
(b) A compound consists of 27% sodium, 16.5% nitrogen and 56.5% oxygen by mass. If its molecular mass is 85g, find:
(i) Its empirical formula
(ii) Molecular formula
10. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
FORM TWO CHEMISTRY EXAM SERIES 143
FORM TWO CHEMISTRY EXAM SERIES 143
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM TWO-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- The valency of an element with atomic number 10 is;
- 2 B. 3 C. 0 D. 1
- How many protons, neutrons and electrons are there in an atom represented by the symbol
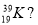
Protons Neutrons Electrons
- 39 19 20
- 19 39 20
- 20 19 20
- 19 20 19
- Separation of a mixture by fraction all distillation is possible if the mixture constituents differ in their;
- Boiling points B. Melting points C. Freezing points D. Vapourizing points
- When element T of Group 1 combines with element X of Group VI, the formula of the compound formed is;
- T2X B. X2T C. TX2 D. XT4
- Saturated solution is one which;
- Contains more solute undissolved at a given temperature
- Has a large amount of solvent at a given temperature
- Contains a little solute at a given temperature
- Will take no more of solute at a given temperature
- The most abundant element on the earth is;
- Carbon B. Iron C. Nitrogen D. Oxygen
- Petrol is an example of;
- Ionic substance C. Flammable substance
- Irritating substance D. Corrosive substance
- Domestic utensils made of iron undergo rusting when exposed to;
A. Air and fire B. Air and oil C. Air and water D. Water and oil
- The chloride iron (Cl-) differs from chlorine atom because chloride ion has;
A. Loss electrons B. Less protons C. More electrons D. More protons
x. Which of the following chemical species have the same number of electrons?
A. Cl, Be, He and O2- B. K+, Ca2+, Cl- and Ar
C. Na+, Mg2+, Al3+ and Li D. O2-, F-, S2- and Cl-
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
SECTION B: 20 MARKS
- You are provided with two lists A and B. Choose a words from list B which matches the statement or phrase in List A and write its letter in the table provided below;
| LIST A | LIST B |
|
|
(b)
SECTION B: 80 MARKS
- (a) What do you understand by the following terms?
- First Aid ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- First Aid Kit ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Name four components which can be found in First Aid Kit;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
(c) Why is it important to provide First Aid to an injured person? Give four reasons;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) For each of the following classes of fire, state the burning material(s);
- Class A fire ……………………………………………………………………………………………
- Class B fire ……………………………………………………………………………………………
- Class C fire ……………………………………………………………………………………………
(b) Fire can be prevented by;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) By giving one example, define the following terms;
- Suspension ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Chromatography ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Briefly explain the following ways of preventing rusting.
- Electroplating ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Galvanizing ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Write the chemical symbols for the following elements;
- Silver ………………………..
- Iron ………………………….
- Aluminium …………………….
- (a) If a mixture of manganese (IV) oxide, MnO2 with potassium chlorate, KClO3 is treated, which of
the two compounds will produce oxygen?
- …………………………………………………………………………………………………
- Why? ………………………………………………………………………………………….
(b) Calculate the oxidation number of the underlined elements;
- NH4+
- SO42-
- CUSO4
- FeCl2
- (a) List down four assumptions of the Dalton’s Atomic Theory;
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
(b) Write a chemical formula for each of the following compounds;
- Water …………………………………………………………..
- Potassium chloride ………………………………………………
- Magnesium oxide ………………………………………………….
- Calcium hydroxide ……………………………………………….
(c) (i) What do you understand by the term “Chemical warning signs”? ……………………………………………………………………………………………………………
(ii) Draw the chemical warning sign that represents;
| HARMFUL OR IRRITANT | TOXIC | FLAMMABLE |
|
|
|
|
- Study the periodic table below;
![]()
![]() I VIII
I VIII
II III IV V VI VII
|
C | A |
D |
|
|
|
|
E |
|
F |
|
|
|
|
G |
H |
|
|
|
J |
|
|
|
|
|
|
Use the letters shown in the periodic table above to indicate;
- Elements with zero valency …………………………
- The lightest atom. …………………………………….
- The alkaline earth match. ……………………………………
- An element with electronic configuration of 2: 8: 1. …………………………..
- Give the names of elements represented by the mentioned letters A, B, C and D. ……………………………………………………………………………………
- Give the name of J as an element. ………………………………………………….
- Write the electronic configuration of J. …………………………………………….
- What type of a bond can be formed when element J combined with element H? ……………………………………………………………………
- What type of a bond can be formed when an element H combines with an element H …………………………………………………………………
- (a) What is air? ………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Mention the composition of air with their percentage composition;
| COMPOSITION | PERCENTAGE (%) COMPOSITION |
|
|
…………………………………….. …………………………………….. …………………………………….. ……………………………………... |
(c) Give four reasons why air is a mixture.
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
iv …………………………………………………………………………………………………
- (a) Give the method used to separate the following mixtures;
- Kerosene and water …………………………………………………………………………………
- Alcohol (ethanol) and water ………………………………………………………………………..
- Iron fillings and sand ……………………………………………………………………………….
- Iodine crystals and table salt ……………………………………………………………………….
(b) Water is a chemical compound? Give four reasons to support this fact.
- ………………………………………………………………………………………………………
- ……………………………………………………………………………………………………….
- ……………………………………………………………………………………………………….
- ………………………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 97
FORM TWO CHEMISTRY EXAM SERIES 97
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRYTERMINALEXAMINATION
FORM TWO-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- The valency of an element with atomic number 10 is;
- 2 B. 3 C. 0 D. 1
- How many protons, neutrons and electrons are there in an atom represented by the symbol
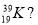
Protons Neutrons Electrons
- 39 19 20
- 19 39 20
- 20 19 20
- 19 20 19
- Separation of a mixture by fraction all distillation is possible if the mixture constituents differ in their;
- Boiling points B. Melting points C. Freezing points D. Vapourizing points
- When element T of Group 1 combines with element X of Group VI, the formula of the compound formed is;
- T2X B. X2T C. TX2 D. XT4
- Saturated solution is one which;
- Contains more solute undissolved at a given temperature
- Has a large amount of solvent at a given temperature
- Contains a little solute at a given temperature
- Will take no more of solute at a given temperature
- The most abundant element on the earth is;
- Carbon B. Iron C. Nitrogen D. Oxygen
- Petrol is an example of;
- Ionic substance C. Flammable substance
- Irritating substance D. Corrosive substance
- Domestic utensils made of iron undergo rusting when exposed to;
A. Air and fire B. Air and oil C. Air and water D. Water and oil
- The chloride iron (Cl-) differs from chlorine atom because chloride ion has;
A. Loss electrons B. Less protons C. More electrons D. More protons
x. Which of the following chemical species have the same number of electrons?
A. Cl, Be, He and O2- B. K+, Ca2+, Cl- and Ar
C. Na+, Mg2+, Al3+ and Li D. O2-, F-, S2- and Cl-
2. (a) You are provided with two lists A and B. Choose a words from list B which matches the statement or phrase in List A and write its letter in the table provided below;
| LIST A | LIST B |
|
|
SECTION B: 80 MARKS
- (a) What do you understand by the following terms?
- First Aid ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- First Aid Kit ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Name four components which can be found in First Aid Kit;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
(c) Why is it important to provide First Aid to an injured person? Give four reasons;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) For each of the following classes of fire, state the burning material(s);
- Class A fire ……………………………………………………………………………………………
- Class B fire ……………………………………………………………………………………………
- Class C fire ……………………………………………………………………………………………
(b) Fire can be prevented by;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) By giving one example, define the following terms;
- Suspension ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Chromatography ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Briefly explain the following ways of preventing rusting.
- Electroplating ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Galvanizing ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Write the chemical symbols for the following elements;
- Silver ………………………..
- Iron ………………………….
- Aluminium …………………….
- (a) If a mixture of manganese (IV) oxide, MnO2 with potassium chlorate, KClO3 is treated, which of
the two compounds will produce oxygen?
- …………………………………………………………………………………………………
- Why? ………………………………………………………………………………………….
(b) Calculate the oxidation number of the underlined elements;
- NH4+
- SO42-
- CUSO4
- FeCl2
- (a) List down four assumptions of the Dalton’s Atomic Theory;
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
(b) Write a chemical formula for each of the following compounds;
- Water …………………………………………………………..
- Potassium chloride ………………………………………………
- Magnesium oxide ………………………………………………….
- Calcium hydroxide ……………………………………………….
(c) (i) What do you understand by the term “Chemical warning signs”? ……………………………………………………………………………………………………………
(ii) Draw the chemical warning sign that represents;
| HARMFUL OR IRRITANT | TOXIC | FLAMMABLE |
- Study the periodic table below;
![]()
![]() I VIII
I VIII
II III IV V VI VII
| C | A | D | E | ||||
| F | G | H | |||||
| J |
Use the letters shown in the periodic table above to indicate;
- Elements with zero valency …………………………
- The lightest atom. …………………………………….
- The alkaline earth match. ……………………………………
- An element with electronic configuration of 2: 8: 1. …………………………..
- Give the names of elements represented by the mentioned letters A, B, C and D. ……………………………………………………………………………………
- Give the name of J as an element. ………………………………………………….
- Write the electronic configuration of J. …………………………………………….
- What type of a bond can be formed when element J combined with element H? ……………………………………………………………………
- What type of a bond can be formed when an element H combines with an element H …………………………………………………………………
- (a) What is air? ………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Mention the composition of air with their percentage composition;
| COMPOSITION | PERCENTAGE (%) COMPOSITION |
| …………………………………….. …………………………………….. …………………………………….. ……………………………………... |
(c) Give four reasons why air is a mixture.
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
iv …………………………………………………………………………………………………
- (a) Give the method used to separate the following mixtures;
- Kerosene and water …………………………………………………………………………………
- Alcohol (ethanol) and water ………………………………………………………………………..
- Iron fillings and sand ……………………………………………………………………………….
- Iodine crystals and table salt ……………………………………………………………………….
(b) Water is a chemical compound? Give four reasons to support this fact.
- ………………………………………………………………………………………………………
- ……………………………………………………………………………………………………….
- ……………………………………………………………………………………………………….
- ………………………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 57
FORM TWO CHEMISTRY EXAM SERIES 57
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- TERMINAL EXAMINATION-MAY
FORM TWO
TIME: 2HRS 2020
NAME:_____________________________________CLASS:___________
INSTRUCTIONS
- Do all questions from sections A, B and C.
- All working must be shown clearly and neatness
- The following atomic masses may be used;
H = 1, C = 12, O = 16, N = 14, Ca = 40, K = 39.
- All writing must be done in black or blue ink EXCEPT for the diagrams which must be in pencil.
SECTION A: 10 MARKS
1. Write down the letter corresponding to the correct answer for each item (i) – (x).
(i) The valency of an element with atomic number 10 is;
- 2
- 3
- 0
- 1
(ii) How many protons, neutrons and electrons are there in an atom represented by the symbol ![]()
Protons Neutrons Electrons
- 39 19 20
- 19 39 20
- 20 19 20
- 19 20 19
(iii) Separation of a mixture by fraction all distillation is possible if the mixture constituents differ in their;
- Boiling points
- Melting points
- Freezing points
- Vapourizing points
(iv) When element T of Group 1 combines with element X of Group VI, the formula of the compound formed is;
- T2X
- X2T
- TX2
- XT4
(v) Saturated solution is one which;
- Contains more solute undissolved at a given temperature
- Has a large amount of solvent at a given temperature
- Contains a little solute at a given temperature
- Will take no more of solute at a given temperature
(vi) The most abundant element on the earth is;
- Carbon
- Iron
- Nitrogen
- Oxygen
(vii) Petrol is an example of;
- Ionic substance
- Irritating substance
- Flammable substance
- Corrosive substance
(viii) Domestic utensils made of iron undergo rusting when exposed to;
- Air and fire
- Air and oil
- Air and water
- Water and oil
(ix) The chloride iron (Cl-) differs from chlorine atom because chloride ion has;
- Loss electrons
- Less protons
- More electrons
- More protons
(x) Which of the following chemical species have the same number of electrons?
- Cl, Be, He and O2-
- K+, Ca2+, Cl- and Ar
- Na+, Mg2+, Al3+ and Li
- O2-, F-, S2- and Cl-
SECTION B: 20 MARKS
2. You are provided with two lists A and B. Choose a words from list B which matches the statement or phrase in List A and write its letter in the table provided below;
| LIST A | LIST B |
|
|
SECTION C: 70 MARKS
- (a) What do you understand by the following terms?
- First Aid …
- First Aid Kit
(b) Name four components which can be found in First Aid Kit;
(c) Why is it important to provide First Aid to an injured person? Give four reasons;
4. (a) For each of the following classes of fire, state the burning material(s);
- Class A fire
- Class B fire
- Class C fire
(b) Fire can be prevented by;
5. (a) By giving one example, define the following terms;
- Suspension
- Chromatography
(b) Briefly explain the following ways of preventing rusting.
- Electroplating
- Galvanizing
(c) Write the chemical symbols for the following elements;
- Silver ………………………..
- Iron ………………………….
- Aluminium …………………….
6. (a) If a mixture of manganese (IV) oxide, MnO2 with potassium chlorate, KClO3 is treated, which of the two compounds will produce oxygen?
- ……………………………………
- Why? ……………………………………
(b) Calculate the oxidation number of the underlined elements;
- NH4+
- SO42-
- CUSO4
- FeCl2
7. (a) List down four assumptions of the Dalton’s Atomic Theory;
(b) Write a chemical formula for each of the following compounds;
- Water ……………………
- Potassium chloride ……… …
- Magnesium oxide …………
- Calcium hydroxide ………… .
(c) (i) What do you understand by the term “Chemical warning signs”?
(ii) Draw the chemical warning sign that represents;
| HARMFUL OR IRRITANT | TOXIC | FLAMMABLE |
|
|
|
|
8. Study the periodic table below;
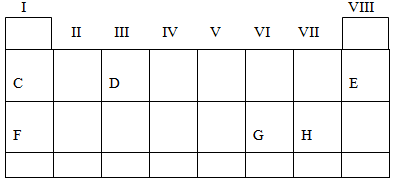
Use the letters shown in the periodic table above to indicate;
- Elements with zero valency ………………
- The lightest atom. ……………………… .
- The alkaline earth match. ………………
- An element with electronic configuration of 2: 8: 1. …………………………..
- Give the names of elements represented by the mentioned letters A, B, C and D. …………………
- Give the name of J as an element. ………………………
- Write the electronic configuration of J. …………………… ……………….
- What type of a bond can be formed when element J combined with element H? ………………………… …
- What type of a bond can be formed when an element H combines with an element H ………………………
9. (a) What is air?
(b) Mention the composition of air with their percentage composition;
| COMPOSITION | PERCENTAGE (%) COMPOSITION |
|
|
(c) Give four reasons why air is a mixture.
10. (a) Give the method used to separate the following mixtures;
- Kerosene and water …………………
- Alcohol (ethanol) and water …………………
- Iron fillings and sand …………………………
- Iodine crystals and table salt …………… …….
(b) What is a chemical compound? Give four reasons to support this fact.
FORM TWO CHEMISTRY EXAM SERIES 15
FORM TWO CHEMISTRY EXAM SERIES 15
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







