CHAPTER 0NE.
NON- METALS AND THEIR COMPOUNDS.
KEY TERM AND CONCEPTS.
- Non-metal- this is an element that reacts by gaining an electron
- Oxidizing property- this is a tendency of a non-metal to gain electron
- Displacement reaction- this is a reaction which a more reactive element displaces a less reactive element from a compound.
- Bleaching property- this is ability of a substance to remove color from another substance either by oxidation or by reduction.
- Frasch process- this is a process in which sulphur is mined from its deposits.
- Allotropy- this is the existence of an element in two or more different physical forms at the same physical state.
- Oleum- this is a compound that is formed when sulphuric acid is dissolved in sulphur trioxide.
- Dehydrating property- this is the ability of conc. Sulphuric acid remove waterand element that make water from other compounds.
- Drying agent property- this is the tendency of a substance called a drying agent to remove water from another substance.
- Fountain experiment- is an experiment designed to illustrate the solubility of ammonia in water.
- Delocalized electrons- these are electrons which are not used in bonding and are free to carry charges.
Meaning of non- metals- are elements which form negative ions by gaining electrons. They react by gaining electrons and the number of electrons an non-metals gains is called its valency.
For example;
Cl+e→Cl-
Chlorine is thus said to be monovalent. All non- metals form negative ions and are called electronegative elements. Thus electronegativity is the ability of an element to form negative ions.
General properties of non- metals.
- Oxidizing properties- all non metals are oxidizing agents because they can accept electrons.
The strength of oxidation differs from one non- metal to another depending on the number of electrons gained.
The oxidation property vary as follows
F>Cl>Br>I>O>S>P>N>C
So fluorine is the strongest oxidizing agent, hence it is said to be the most electronegative element.
Displacement of non-metal by another non- metal from a compound.
Depending on oxidizing power, non- metals can displace each other from their solutions. Chlorine displaces both bromine and iodine from their compounds, while bromine can displace iodine from its compound.
Chlorine + hydrogen bromide→hydrogen bromide + bromine gas.
Cl2(g)+HBr→HCl+Br2
With sodium bromide, bromine gas is liberated from the solution as follows
chlorine + sodium bromide →sodium chloride + bromine
Cl2+ 2NaBr →2NaCl + Br2
Chlorine reacts with potassium iodide to get potassium chloride and iodine
![]() Cl2 +2KBrBr2 + 2KCl
Cl2 +2KBrBr2 + 2KCl
In the same way bromine displaces iodine ions from potassium iodide and iodine is formed.
When chlorine is bubbled through potassium iodide, the clear solution turns dark brown and a dark solid is deposited.
Chlorine.
Chlorine is a very reactive gas and hence it does not occur freely in nature. It is found combined with other elements e.g sodium forming sodium chloride.
Preparation of chlorine gas in the laboratory.
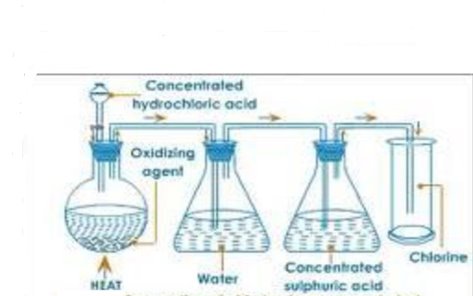
Figure 1.1 preparations of chlorine
It is prepared by action of concentrated hydrochloric on an oxidizing agent. The oxidizing agent provides the oxygen atoms needed to oxidize the acid. The potassium permanganate is placed in the flask and arranged as shown above.
When the acid comes into contact with the permanganate, effervescence occurs and a greenish – yellow gas is given off. The hydrogen chloride produced together with chlorine dissolves in water. The chlorine gas is passed through concentrated sulphuric acid in order to be dried out. It is collected by downward delivery because it is denser than air.
Eqn- hydrochloric acid + potassium permanganate→potassium chloride + managanese chloride + water + chlorine.
16HCl(aq) + 2KMnO 4(s) 2KCL (aq) +2MnCl 2(aq) + 8H2O(l) + 5Cl2(g).
Chemical properties of chlorine gas.
- Action on metals.
It reacts with all metals to form metallic chloride.
Examples include;
Alluminium + chlorine → Aluminium chloride
2AI(s) + 3Cl 2(g) →2AlC 3(aq)
Sodium + chlorine → sodium chloride
Na (s) + Cl 2(g) → NaCl(s)
- Bleaching Action.
Chlorine bleaches damp litmus paper and wet flowers. This shows that chlorine is a bleaching agent. The gas first reacts with water to form a mixture of hydrochloric acid and chloric I Acid- hypochlorous acid.
Chlorine + Water → hydrochloric acid + hypochlorous Acid
Cl2(g) + H20(l) → HCL(aq) + HOCl(aq)
Chloric I acid is unstable and hence decomposes to form hydrochloric acid and an oxygen atom.
HOCl(aq) →HCl(aq) + ( dye + [o] ) –colorless.
The colured flowers are decolourised after some time.
Hydrogen chloride can exist in gaseous form i.ehydrogen chloride gas and hydrochloric acid.
- Chlorine as an oxidizing agent.
Chlorine reacts by accepting electrons,
Cl2(g) + 2e(aq) → 2Cl-(aq)
- With hydrogen sulphide
Cl2 oxidizes hydrogen sulphide to Sulphur at room temperature while itself is reduced to chloride ion
Hydrogen chloride + chlorine→ hydrogen chloride + Sulphur
H2S(g) +Cl2(g) →2HCl(aq)+S(s)
- With Sulphur (iv) oxide
Chlorine oxidises Sulphur IV oxide to sulphuric (VI) Acid.
SO2(g) + 2H2O(l) + Cl 2(g) → H2SO4(aq) + 2HCL(aq)
When Sulphur IV Oxide is passed into water, sulphorous acid is formed. This acid is converted into sulphuric acid when chlorine is blown over it.
- With iron (II) chloride.
Iron (II) chloride is oxidized into iron (III) Chloride
2FeCl 2(s) + Cl2(g) → 2FeCl 3(s)
Uses of chlorine.
It is used as;
- Bleaching agent
- Germicide and weed killer
- Manufacture of hydrogen chloride gas
- Manufacture of plastics
- In water treatment
- For treatment of sewage,
- As a disinfectant
Test for chlorine gas.
It is greenish –yellow in color gas which rapidly bleaches a damp litmus paper.
Hydrogen Chloride Gas.
Preparation
This gas is prepared by reacting concentrated sulphuric acid with sodium chloride in a round bottomed flask. Sodium chloride is also called a rock salt. The apparatus for the preparations are shown below;
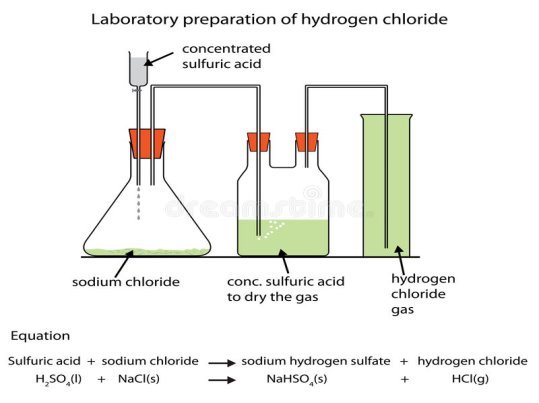
Figure 1.2 preparation of hydrogen chloride.
Eqn. sodium chloride + Sulphuric Acid → sodium hydrogen sulphate + Hydrogen Chloride
NaCl (s) + H2SO4(aq) → NaHSO4(aq) + HCl(aq).
The gas is dried by passing over concentrated Sulphuric acid
Physical properties of the gas.
- It is colourless
- It is denser than air
- It is very soluble in water
Chemical properties of hydrogen chloride gas
- It is an acidic gas, thus turns litmus paper red,
- It does not support combustion
- When exposed in air it forms white fumes
- It is higly soluble in water
- Reacts with ammonium to form white fumes of ammonium chloride.
HCl (g) + NH 3(g)→ NH4Cl(s)-white fumes.
The diagram below shows how the gas can be dissolved in water due to its solubility.
- It reacts with metals to from hydrogen gas and forming hydrogen chloride.
Hydrochloric acid.
When hydrogen chloride gas is dissolved in water, hydrochloric acid is formed. In the industry, this acid can be manufactured by reacting hydrogengas and chlorine gas. Hydrogen is obtained from cracking of alkanes, while chlorine is obtained from the down cell. The two gases are reacted at very high temperature as shown in the equation below.
H2(g) + Cl2(g) → 2HCl(g).
To avoid explosion a small amount of hydrogen is allowed to burn in a jet. The product is dissolved in water to form hydrochloric acid which is 35% pure.
Uses of HCl acid.
- Removing rust from iron
- Chlorination of water
- Sewage treatment
- Manufacture of silver chloride used in photographic films.
SUMMARY ON CHLORINE.
- Chlorine is found in group seven of the periodic table and is called an halogen
- It is a very reactive element
- Can be prepared in lab using HCL and a catalyst
- The gas is acidic. Greenish yellow, has a chocking smell and very poisonous
- In industry, chlorine is manufactured in large scale by reacting hydrogen and chlorine
- Hydrogen chloride, a compound of chlorine is manufactured by reacting conc. HCl and sodium chloride mixed with conc. Sulphuric acid.
- The solution of hydrogen chloride in water is called hydrochloric acid.
- Chlorine has a wide applications in the industry.
CHLORINE QUESTIONS
- I.Explain how chlorine water can be formed.
Ii. Which Acid of HCL/HOCL is a bleaching agent
- Write an equation to show bleaching action of hypochloilsa acid.
- Explain using equation how the presence of chloride ions can be identified in
- Solid
- Solution
- Study the diagram below and answer the questions that follow
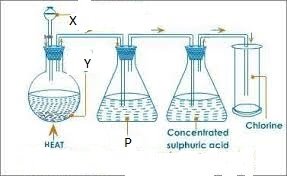
The apparatus above are used to prepare chlorine
- State substance
Y
X
P
- What is the use of conc. Sulphuric acid?
- State the use of p.
- Write equation for reaction occurring at the flask.
- How can you show that test tube used for collection of chlorine is full?
- Name the method of collection
- Give two uses of chlorine
- State 2 compounds of chlorine that pollute the environment.
- Write equation for reaction between
- Chlorine and Magnesium
- Chlorine and phosphorous
- Chlorine and copper
- Chlorine and hydrogen Sulphate
- Explain what happens when a stream of chlorine gas is passed over
- Hydrogen sulphuric gas
- Iron II solution.
Write equation for the reaction.
- Explain how chlorine water can be formed.
Ii. Which Acid of HCL/HOCL is a bleaching agent
- Write an equation to show bleaching action of hypochlorous acid.
- Explain using equation how the presence of chloride ions can be identified in
- Solid
- Solution
- Write equation for reaction between
- Chlorine and Magnesium
- Chlorine and phosphorous
- Chlorine and copper
- Chlorine and hydrogen sulphate
- a)Explain what happens when a stream of chlorine gas is passed over
- Hydrogen sulphuric gas
- Iron II solution.
b) Write equation for the reaction.
- Ammonium sulphate is fertilizer produced by passing ammonia gas into concentrated sulphuric acid. Write down an equation for the reaction.
SAMPLE NECTA QNS.
2004.
6. Figure 1 below represents the laboratory preparation of hydrogen chloride gas.
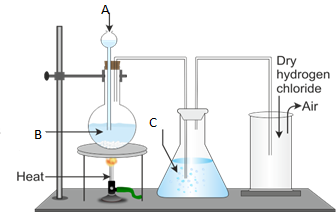
(a) Name the parts labelled A, B, C and D.
(b) (i) Do you think the gas can be collected over water? Give reasons for your answer.
- Explain the test for the gas.
- What is the function of C?
- Name the method used to collect the gas.
- Write a balanced chemical equation for the reaction taking place during the preparation of hydrogen chloride gas.
(c) Write chemical equations for the reaction between:
(i) Ammonia gas and hydrogen chloride.
(ii) Hydrogen chloride gas and water.
- The reaction between hot concentrated sodium hydroxide and chloride gas produces sodium chlorate (V), Sodium chloride and water.
- Write down an equation for the reaction.
- Give one use of sodium chlorate (V).
- The following experiments were carried out chloride water contained in two test tubes.
- A blue flower petal were carried out on chlorine in two test tubes.
- The second test tube was corked and exposed to sunlight. After a few days, it was found to contain a gas that rekindles a glowing splint. Write down an equation for this reaction.
NITROGEN AND ITS COMPOUNDS.
Nitrogen is a non metal in group V of the periodic table. It is a gas at room temperature and relative unreactive.
Occurrence.
Nitrogen occurs in free state in the atmosphere. It accounts for 78% of the atmosphere. It is also occur in combined form of chile salt petre (NaNO3), as a mineral deposit in chile. It also occurs in minute quantities in compoinds like ammonium sulphate and sodium nitrate. Nitrogen is an important constituent of living matter.
Obtaining nitrogen from the atmosphere.
Nitrogen occurs in free state in the atmosphere, and can be separated by removing all other constituents. The apparatus below shows how it can be separated.
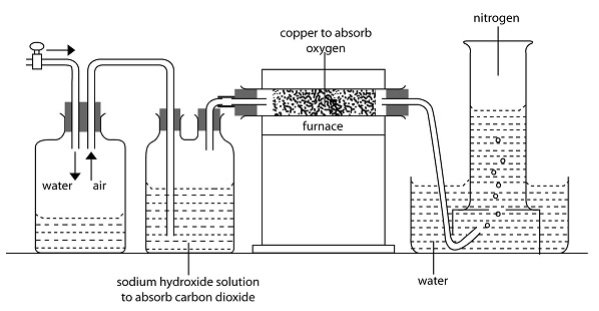
The water is used to drive off air. It passes, through conc. Sodium, hydroxide which absorbs carbon IV oxide. Its then passes over, heated copper metal. The oxygen is removed. The remaining air is nitrogen which of course is not pure because of traces of rare gases.
2NaOH(aq) + CO 2(g) →Na2CO 3(aq) + H2O(l)
Absorption of oxygen
2Cu(s)+O2(g) → 2CUO(s)
The presence of noble gas make atmospheric nitrogen denser than pure gas.
Nitrogen can also be prepared in the laboratory by heating ammonium nitrate
NH4NO3 +NH4Cl→NaCl+NH4NO2
Properties of Nitrogen.
- It is colourless, odourless, and insoluble in water
- It tests negative for all common test for gases
- It reacts with nitrogen under special conditions.
- It can react with burning magnesium to form magnesium nitride
3Mg(s) + N2(g) → MgN3(s)
When magnesium nitride is reacted with water, ammonia gas is formed.
Mg3N2+ H2O(l) → 3Mg(OH)2+2NH3(g).
Uses of nitrogen.
- In manufacture of ammonia by haber process
- Makes atmospheric oxygen inert
- Is a source of fertilizer especially when fixed by bacteria
- Used as refrigerant in semen preservation
- Used in light bulb to prevent the oxidation of filament
Ammonia
It is a compound of hydrogen and nitrogen. It has a pair of electrons thus making it highly soluble.
It is a gas with a pungent smell.
Preparation of ammonia gas.
It is prepared by reacting ammonium gas with an alkali.
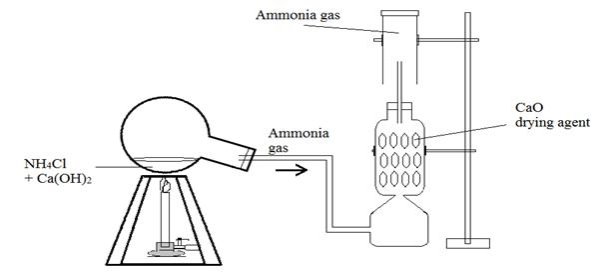
Figure 1.3 preparation of ammonia
Ca(OH)2(aq) + NH4Cl (s) → CaCl2(s) + 2 H2O(l) + 2NH3(g)
The ammonia is dried by use of calcium oxide.Ammonia reacts with many common drying agent like sulphuric acid and calcium chloride
2NH 3(g) + H2SO4(l) → (NH4)2SO4(s)
Ammonia is less denser than air and is collected by upward delivery.
Physical properties of ammonia gas.
- Is colorless gas.
- It is less dense than air
- Has a chocking pungent smell
- Highly soluble in water
Chemical properties of ammonia gas.
- It is an alkaline gas, thus turns moist litmus paper blue
- Reacts with hydrogen chloride to form white fumes of ammonium chloride- this is the chemical test for ammonia gas.
- Ammonia gas is highly soluble in water as shown by the fountain experiment.
NH 3(g) + H2O(l) → NH+ + OH-
The fountain experiment.
This is an experiment that is used to show how soluble in water ammonia gas is. A hard round bottomed flask is fitted with two tubes each with clips as shown below. It is then immersed in a water bath half full with water. 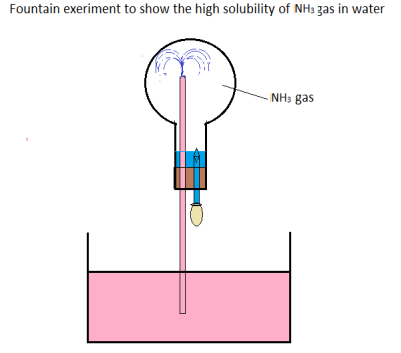
Figure 1.4 The fountain experiment
A little litmus solution is added to the water in trough. One of the taps is opened and a drop of water enters into the flask. This dissolves a great volume of the gas, that a partial vacuum is created. When the other tap is opened, water is forced up the flask until it is full of water, the solution turns blue because ammonia is basic.
Reactions of ammonia with acids.
Ammonia being an alkali reacts with acids to form a salt and water only. This is neutralization reaction.
NH 3(g)+ H2SO4(aq) → (NH4)2SO4
Combustion of ammonia
In excess of oxygen, it forms water and nitrogen gas.
NH 3(g) + 3O2(g) → 2N2(g) + 6 H2O(g)
In presence of a catalyst, such as hot platinum wire, ammonia reacts to form nitrogen II oxide and water. The NO is oxidized to brown nitrogen dioxide.
Reaction with copper oxide.
Ammonia reduces copper II oxide to form brown copper metal, nitrogen gas and water.
CuO(s) + NH 3(g) →Cu(s) + N2(g) + H2O.
THE HABER PROCESS.
This is an industrial manufacture of ammonia gas. It is based on direct combination of nitrogen gas and hydrogen gas. The hydrogen comes from cracking of alkanes, while the nitrogen is obtained by separation of nitrogen gas from air through fractional distillation.
N2(g) + 3 H 2(g)→2NH3g + ∆-ve
The reaction takes place in the following condtions;
- High pressure of about 200-500atm
- Low temperature of about 450Oc
- Use of iron catalyst
The process.
A mixer of nitrogen and hydrogen gas is passed through a purifier to remove impurities which may poison the catalyst. The mixer is then passed through a compressor at a pressure of 500atm. The two gases combine as follows; N2(g) + 3 H2(g) →2NH3g) + ∆-ve
The hot mixer is passed through heat exchanger where it is cooled. It is then passed through a catalytic chamber where the uncombined gases are combined. The ammonia condenses and is collected as a liquid. This process has no dangerous by- products as the uncombined gases are recycled.
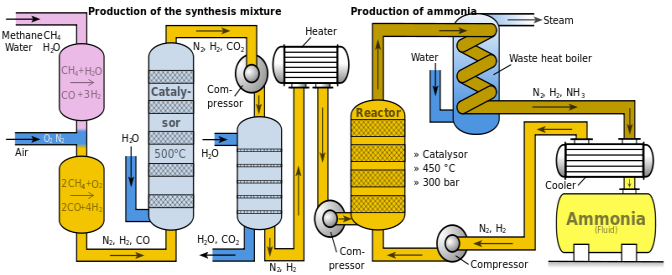
Figure 1.5 The haber process
Uses of Ammonia gas.
- Ammonia solution is used in laundry work. It removes temporary hardness of water.
- Dissolves acids in under cloths thus help to remove stains
- Used in the manufacture of nitric acid
- Used as a refrigerant in big warehouses
- Manufacture of hydrazine which is used as a rocket fuel
- Used in softening of water.
SUMMARY.
- Nitrogen is a colourless gas that is unreactive
- It occupies 78% of the atmosphere
- It can be obtained from the air by isolation
- Ammonia can be manufactured by combining hydrogen and nitrogen under special conditions.
- Ammonia is alkaline and highly soluble in water
- Nitrogen gas has many uses in the industry including in refrigerations.
- Nitrogen is a colorless gas, very unreactive it is in group seven accounting for 78% of atmosphere.
- Can be obtained from air by isolation
- Can also be prepared by decomposition of ammonia nitrate.
- Main oxide of nitrogen are
Nitrogen I oxide
Nitrogen II oxide
Nitrogen III oxide
- Nitrogen I oxide has sweet sickly smell and support burning
- Nitrogen (IV) Oxide is reddish brown gas
- Ammonia can be manufactured by combining nitrogen and hydrogen under special conditions. i.e
- Pressure of 200 – 500 tm
- Temperatureof 4500C
- Iron Catalyst
- Ammonia is used to make fertilizers and nitric acid
- Ammonia is alkaline and a highly soluble in water it is the most soluble gas in water
- Nitric acid is manufactured by catalystic oxidation of ammonia
- Nitric acid is a fuming gas, which attacks and comments most things except glass.
- Nitrates are affected by heat depending on reactivity of the metal.
- All nitrate are sources in water
- Nitrate can be identified by brown ring test.
END OF TOPIC QUESTIONS.
- i. Explain how a sample of nitrogen can be isolated from air
ii. Ammonia is manufactured by water process. Name the best conduct for this process, and state 2 uses of ammonia
- Explain how nitrogen can be prepared in laboratory
- Explain the observation made.
- Brown former are observed when NO is exposed to air
- Nitric acid is stored in dark brown bottles
- Nitric acid is often yellow.
- Given NH4(SO4)2 and NH4NO3 Which one would you say is the best fertilizer? Show your working
Calculate the volume of oxygen produced when 20g KNO3 is is decomposed. Molar gas vol at composed. Molar gas vol at stp = 22.4 liter
- Using equation show and name reagent used to make
- Nitrogen
- nitrogen oxide
- Nitrogen dioxide gases in laboratory
- When a small burning Magnesium ribon was lowered into jar nitrogen dioxide, a white powder was product and brown colour disappeared. Suggest explain for the observation
b . The equation below is for manufacture of ammonium by Haber process
N2 (g) + 3H2 (g)⇋2NH3∆H = -Ve
- In term of reacting volume what is the best pressure for the reaction above.
- Does it require high or the low temperature?
- State how the oxidation of ammonia gas by burning in oxygen differs from it catalytic uses platinum as the catalyst.
- The set up below shows the preparation of nitrogen gas. Study it and answer questions that follows.
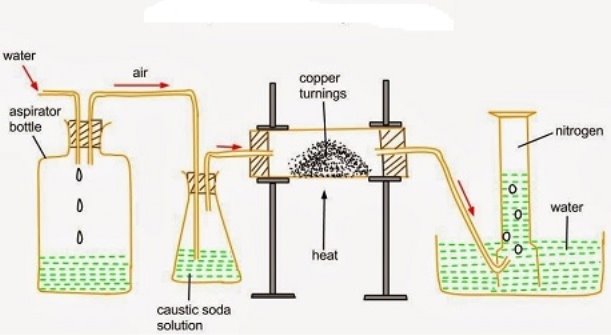
- State the purpose of sodium hydroxide
- Write down an equation that shows the reaction taking place in the round bottomed flask
- With the aid of an equation, explain what takes place in the combustion tubes.
- The nitrogen obtained using this is not pure. Explain why this is so
- During the haber process of the manufacture of ammonia, hydrogen and nitrogen rreact according to the equation show below.
N2 + 3H2 ⇋2NH2(g) (H = -92Kj)
- Name the catalyst used in above process.
- Calculate how much energy would be evolved it 10g of hydrogen is used (H = 1.0).
SAMPLE NECTA QUESTIONS.
3. (a) Write ionic equations for the following:
- Laboratory preparation of ammonia gas.
- Precipitation of barium sulphate from barium chloride and sodium sulphate and silver chloride from a soluble chloride.
- Neutralization of a strong acid and a strong alkali.
2007.
QN5 © (c) What will happen when:
- yellow flowers are introduced into a gas containing chlorine gas?
- a burning splint is introduced into a gas jar containing hydrogen gas?
- a glass rod which was dipped in concentrated hydrochloric acid is introduced into a gas jar containing ammonia gas?
- sulphur dioxide gas is bubbled through a yellow acidified potassium dichromate solution?
2009.
QN. 11.The preparation of ammonia in the laboratory is done by heating a mixture of ammonium chloride and sodium hydroxide.
(a) (i) Write a balanced chemical equation for the above reaction.
(ii) Using balanced chemical equations, state how ammonia reacts with hydrogen chloride gas and heated copper (II) oxide.
(b) (1) State two uses of ammonia.
(ii) Name the catalyst used in the preparation of ammonia.
(c) Explain each of the following reactions, giving observations and equations.
- Aqueous ammonia is added to iron (III) chloride, little by little, until in excess.
- Sodium nitrate is strongly heated.
QNS. 8. (a) Ammonia gas can be prepared by heating an ammonium salt with an alkali.
- Name the most common pair of reagents suitable for this reaction.
- Write the equation for the reaction.
(b) Ammonia is very soluble in water and less dense than air. How does each of the properties determine the way in which ammonia is collected in a gas jar?
(c) Give reasons for the following:
- A solution of chlorine in water is acidic.
- Yellow phosphorus is stored under water.
QUESTIONS AND ANSWER SECTION.
1. (a) Write the electronic configuration, group and period of nitrogen
(b) Explain how nitrogen is obtained in large scale
© List three properties of nitrogen
(d) Mention three uses of nitrogen
(e) Mention the ways of obtaining pure nitrogen.
Solution.
(a) N = 2:5
Group V
Period 2.
(b) Properties of N2
- Colourless and odourless
- Little less denser than air
- Almost insoluble in water
- Generally unreactive
© Uses of nitrogen
- Manufacture of ammonia
- Provide inert atmosphere
- Air refrigerant
- In light bulb
(d) Obtaining pure nitrogen;
- Heating ammonia nitrate
NH4NO2→2H2O + N2(g)
- Oxidation of ammonia
2NH3(g) + 3CuO→Cu(s) + 3H2O + N2(g)
2.the set up below is used in the preparation of ammonia gas
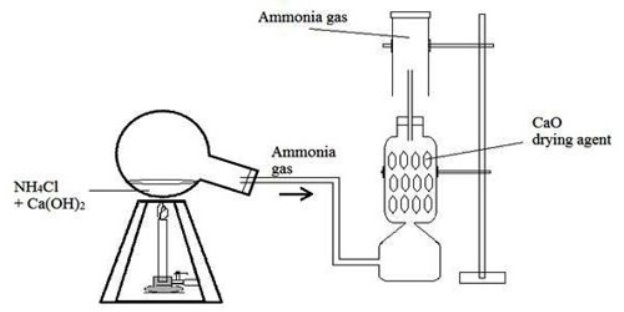
- Why is the flask with the mixture in a tilting position?
- How can you tell the test tube is full of the gas
- Why are usual drying agents, calcium chloride and concentrated sulphuric acid not used.
- Write the equation for the reaction between;
- Ammonium chloride and calcium hydroxide
- Hydrochloric acid and ammonia
- Ammonia and water
v)state the observations made when;
- A test tube full of ammonia gas is inverted
- Glass rod dipped in conc. Sulphuric acid is brought near mouth of test-tube full of ammonia.
Solution.
- To prevent the water which condenses on the cooler parts of the apparatus from running back into the flask.
- Ammonia if present would turn wet litmus paper blue for it is the only alkaline gas.
- Ammonia reacts with common drying agents
CaCl2(s) + 4NH3(g)⇋CaCl2.4NH3(s)
H2SO4(l) + 2NH3(g)→ (NH4)2SO4(aq)
iv)Ca(OH)2+ 2NH4Cl(s) →CaCl2(s) + 2H2O(l) +2NH3(g)
b) HCl (aq) + NH3(g) →NH4Cl(s)
c) NH3(g)+H2O(l) →NH4OH(aq)
v) (a) Ammonia would dissolve in water suddenly and the space taken over by the ammonia solution.
b) A glass rod dipped into concentrated hydrochloric acid was brought near a mouth of a test-tube with ammonia gas
NH3(g)+HCl(g) ⇋NH4Cl(s)
5. The following equation shows the reaction which occurs during the industrial manufacture of ammonia gas.
N2(g)+3H2(g) ⥧ 2NH3(g)∆H = -92KJ.
i)Explain why pressure of 250 atm and a temperature of 4500C is normally used in the above process.
ii) name the catalyst used and its role
iii) the percentage conversion of nitrogen and hydrogen into ammonia is 8%, explain how ammonia can be separated from unreacted gases.
iv) ammonia can be catalytically converted into nitric acid; write equation showing how this conversion is possible.
Solution.
i) A high pressure favours a forward reaction since the reaction takes place with decrease in number of moles.
Because the reaction is exothermic, low temperature would favour production of ammonia, but since at low temperature reaction is too slow, high temperature is used.
ii) finely divided iron catalyst to speed up the reaction rate
iii) ammonia is liquefied by refrigeration
iv) 4NH3(g)+ 5O2(g) →4NO(g) + 6H2O(l)
2NO(g) + O2(g) →2NO2(g)
4NO2(g)+ 2H20 + O2(g)→ 4HNO3(aq).
5. Ammonia is manufactured in large scale by haber process,
(a) Name raw materials for manufacture of ammonia and their sources
(b) Describe chemical reaction and conditions for manufacture of ammonia
© According to le chateliers principle, what conditions should be adopted for maximum yield of ammonia?
(d) how is ammonia gas isolated for storage?
(e) explain what happens to gases that remain unreacted at the end of the reaction
(f) state the uses of ammonia gas.
Solution.
(a) – hydrogen from electrolysis or from methane
- nitrogen – from fractional distillation of air.
(b) nitrogen and hydrogen are mixed in a ratio of 1:3 and reacted together at high pressure of 250atm over finely divide iron catalyst at a temperature of 500Oc. They react to form ammonia as follows;
3H2(g)+ N2(g)→ NH3(g) + ∆H = -92Kj
c) four volumes of the reactant forms two volumes of product. Increase in pressure will favour the forward reaction since this reaction is accompanied by a decrease in volume, hence high pressure should increase the yield of ammonia and also increase the rate of reaction as collision frequency increases. Forward reaction is exothermic hence a decrease in temperature will favour forward reaction.
(d) How is ammonia gas isolated for storage- the ammonia is removed as aqueous solution by washing with water or condensed as a liquid by refrigeration at -20
(e) The unreacted hydrogen and nitrogen and uncondensed ammonia are recycled.
(e) Uses of ammonia gas
- Liquid ammonia is used as a lubricant
- Manufacture of nitrogeneous fertilizer
- Manufacture of nitric acid and urea
- Soften water for domestic use and remove greasy
- Used in solvay process to manufacture sodium carbonate
- Production of synthetic fibre like nylon
6.What is catalytic oxidation of ammonia?
Solution-
This is the oxidation of ammonia by oxygen which takes place on the surface of metallic catalyst eg. Platinum with evolution of heat.
Oxidation process is a twin process;
4NH3(g)+ 5NO2(g) →4NO(g) + 6H2O(s)
2NO(g) + O2(g)→ 2NO2(g)
SULPHUR AND ITS COMPOUNDS.
OCCURRENCE.
Sulphur occurs in form of mineral compounds such as
- Pyrite- Fes
- Gypsum – CaSO4-2H2O
- Mirabilite- Na2SO4.10H2O.
Sulphur is also a constituent of plant and animal protein. Elemental Sulphur is not toxic to humans but it has fungicidal effects.
Pure Sulphur is fragile, crystallined, yellow in color, with several allotropic modifications. These allotropes include, rhombic, monoclinic, plastic, and prismatic.
Sulphur occur in rings called Sulphur 8 rings.
Extraction of Sulphur.
Sulphur is obtained from underground by a process called frasch process. The Sulphur is found about 200m underground. Three concentric rings are erected downward to the molten Sulphur.Super heated water at 170oC, IS passed through outer pipe, where it melts the hot Sulphur. Hot air is passed at 16atm, through middle pipe and helps in making the Sulphur molten. Because of the pressure of the build inside; molten Sulphur is passed through middle ring (pipe) and is collected in a reservoir.
Diagram.
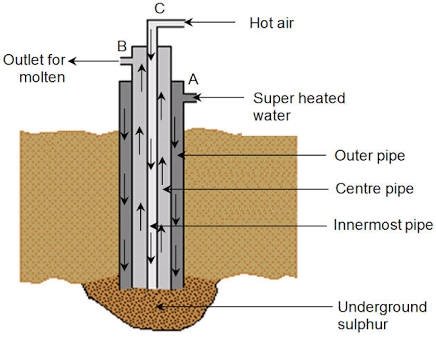
Figure 1.6 Frasch process
Allotropes of Sulphur.
Sulphur exists in two main allotropes,
Rhombic and monoclinic
Other allotropes includes plastic and amorphous Sulphur.
Rhombic Sulphur.
Also called alpha Sulphur or octahedral Sulphur due to the shape of its crystals. It is formed when Sulphur is dissolved in warm methyl benzene. The shape of rhombic Sulphur is as shown below.
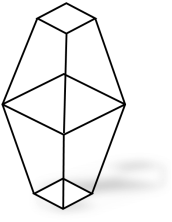 Figure 1.7 Rhombic sulphur
Figure 1.7 Rhombic sulphur
rhombic Sulphur is stable below 96oc.
Monoclinic Sulphur.
It is also called prismatic Sulphur or beta Sulphur. It is formed when Sulphur is heated slowly on evaporating dish until it melts. Monoclinic Sulphur is stable above 960C.
 Figure 1.8 monoclinic sulphur
Figure 1.8 monoclinic sulphur
Plastic Sulphur.
Formed when molten Sulphur is gradually poured in a beaker of cold water. At room temperature plastic Sulphur gradually changes into rhombic Sulphur.
The temperature of 960C, above which monoclic Sulphur is formed and below which rhombic sulphur is formed is called transition temperature.
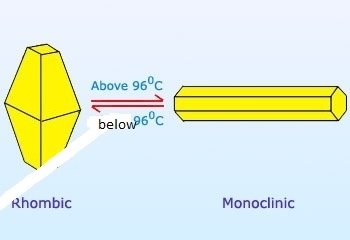
Figure 1.9 Transition between the two allotropes
Effects of heat on Sulphur.
- At 119oC, SULPHUR melts to give a yellow liquid whose viscousity decreases until 1550C. This is because of breaking S-S rings.
- On further heating, viscosity increases, upto 159oc due to opening of S8 rings
- Above 200Oc, viscosity with the liquid eventually acquiring a more reddish hue at high temperature. This is caused by thermal breakingof Sulphur chains
- The liquid boils at 444oc to form a reddish brown vapour.
Properties of Sulphur.
- Combustion
Burns in plenty of air to from Sulphur dioxide, in air enriched with oxygen, it burns to form Sulphur trioxide.
S (s)+02(g)→SO2(g)
2S(s) + O2(g)→ 2S03(g)
Reaction with metal.
Sulphur reacts with iron and copper to form sulphides.
Fe(s) + S(s)→ FeS(s)
Fe(s) + Cu(s)→ Cu2S(s)
Both iron sulphide and copper sulphide are black in color.
Reaction with non metal
With cabon, Sulphur combines to from carbon disulphide which is very poisonous.
Cs+S s →CS2g
Reactions with acids.
With nitric acid, sulphuric acid is formed and the nitric acid is reduced to brown nitrogen IV Oxide.
S(s) + 6HNO3(aq) → H2SO4 + NO2 + 2H2O
Concentrated sulphuric acid oxidizes Sulphur into Sulphur dioxide and water.
H2SO4(l) + S (s)→ 3S02(g) + 2 H2O(l).
Hydrochloric acid does not behave the same way because it is not an oxiziding agent.
Uses of Sulphur.
- As a fungicide in dusting of vines
- Manufacture of sulphuric acid in contact process
- Vulcanization or hardening of rubber
- Making bleaching agents in the paper industry
- Manufacture of fireworks, dyes and Sulphur compounds.
The contact process.
This is the industrial manufacture of sulphuric acid. The process involves four stages which are;
- Preparation of Sulphur IV Oxide
- Purification of Sulphur IV oxide
- Conversion of Sulphur IV Oxide into Suphur VI Oxide.
- Conversion of Sulphur (VI) Oxide into sulphuric VI acid.
- Preparation of Sulphur IV Oxide
Formed by burning Sulphur in air or burning Sulphur ores in air.
- S(s) + O 2(g)→ SO2(g)
- 2Fe S(s) + O 2(g)→ SO2(g)
Other sulphides such as zinc, copper, can be used instead of iron.
- Purification of Sulphur (IV) Oxide
The mixture of Sulphur(IV) Oxide and excess air are purified to remove dust particles which may poison the catalyst.
- Conversion of Sulphur dioxide into Sulphur trioxide.
This requires a pressure of 2-3atm, temperature of 400-500Oc and a vanadium (V) Catalyst.
This reaction takes place in a catalytic chamber.
2S02(g) + O2(g)→2S03(g) heat= -94KJ/mol
- Conversion of Sulphur (VI) Oxide into sulphuric (VI) Acid.
The Sulphur VI oxide is passed over absorption tower where it reacts with concentrated sulphuric VI Acid to form oleum,
Sulphur(VI) Oxide + Sulphuric (VI) acid→ Oleum
SO 3(g)+H2SO4(l)→ 2 H2S20 7(l)
The oleum formed is then diluted with water to give concentrated sulphuric acid. This occurs in dilution chamber
Oleum + water → Sulphuric (VI) Acid
H2S2O 7(l)+H2O(l) → H2SO4(l)
DIAGRAM FOR CONTACT PROCESS.
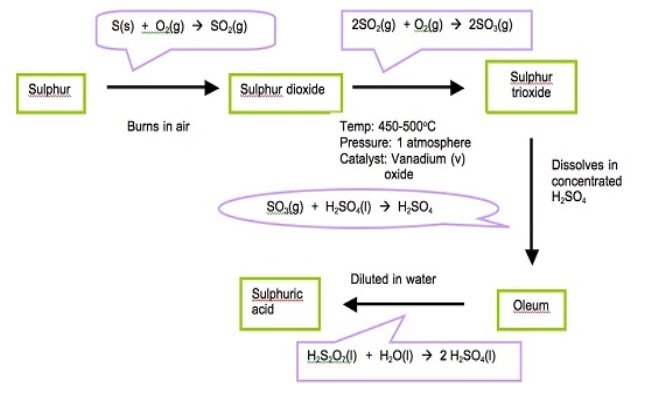
Figure 1.10. The contactprocess
Chemical properties of concentrated sulphuric acid.
- Dehydrating property
This is the ability of the acid to remove elements of water, that is hydrogen and oxygen from a compound.
When conc. Sulphuric acid is added to sugar in a bench, the acids removes oxygen and hydrogen leaving a black mass of carbon.
C12H22011(s)![]() 12C + 11H20
12C + 11H20
Crystals of blue copper II Sulphate turns white when Conc-sulphuric acid is added.
CUSO4.5H2O(s)![]() CuSO 4(s)+5H20(l)
CuSO 4(s)+5H20(l)
- Drying agent
The acid is hygroscopic, it absorbs water from the atmosphere and it becomes diluted. When left in open, the volume of the sulphuric acid is found to have increased.
- Oxidizing power of Conc Sulphuric acid.
It oxidizes zinc and copper to form their salts. Sulphur dioxide gas is also liberated.
Cu(s) + H2SO4(l) → CuSO4(aq) + 2H2O(l) + SO2(g)
Zn(s) + H2SO4(l)→ZnSO4(aq) + 2H2O(l) + SO2(g)
The acid oxidizes non-metals to their 0xides
C(s) + H2S04(l) → CO2(g) + S0 2(g) + H20(l)
S(s) + H2S04(l)→S02(g) +2 H20(l)
Dilute Sulphuric acid.
- Reaction with metals
It reacts with metals above hydrogen liberating hydrogen gas. For very reactive metal, the reaction is very dangerous and should not be tried.
The reactivity decreases as one moves down the reactivity series.
Eg. Mg (s) + H2S04(aq)→ MgSO4(ag) + H2(g)
Reaction with carbonate and hydrogen carbonate.
The product of this reaction is carbon dioxide salt and water. The presence of carbon dioxide can be confirmed by use of lime water where it turns into white precipitate.
The reaction between calcium and lead with sulphuric acid stops after a certain period of timw due to formation of insoluble salts.
Na2CO3(s) + H2SO4(aq)→Na2SO4(aq) + CO2(g) + H20(l)
ZnCO3(s) + H2SO4(aq)→ZnSO4(aq) + CO2(g) + H20(l)
CuCO 3(s) + H2SO4(aq)→CuSO4(aq) + CO2(g) + H20(l)
Reaction with hydroxides of metals.
It produces salts and water with hydroxides and metal oxides, this is called neutralization.
Copper oxide reacts with dilute sulphuric acid to form a blue solution of copper II Sulphate.
CuO(s) + H2SO4(aq) →CuSO4(aq) + H20(l)
With copper hydroxide,
Cu(OH)2(s) + H2SO4(aq) →CuSO4 + H2O
USES OF SULPHURIC ACID
Sulphuric acid is used in the following ways.
- Manufacture of fertilizers
- Making synthetic fibres
- In refining petroleum
- Petro chemicals and dye stuffs
- Pigments of paints
- In metallurgy sulphuric acid is used in extraction of metals
- In car batteries and accumulators
- As drying agents for gases
- In pickling; cleaning of metal surfaces
- In the manufacture of soaps and detergents.
Sulphur (IV) Oxide.
This gas is prepared in the laboratory by reactingconc. H2SO4 acid and copper metal. The gas is dried over conc. Sulhuric acid and collected by down ward delivery. It has irritating smell and is colorless.
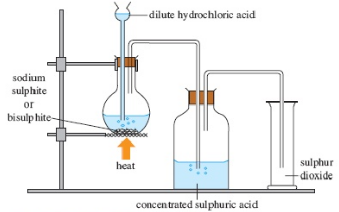
Figure 1.11 Preparation of sulphur dioxide
Properties of Sulphur IV Oxide.
Physical properties.
- It is a colorless gas with an irritating smell.
- It turns damp litmus paper blue and then bleaches it.
- It is denser than air
- It is readly soluble in water forming sulphorous acid.
Chemical properties.
- The gas neutralizes alkalis such as sodium hydroxide forming sodium hydrogen sulphite and water.
NaOH(aq) + SO2(g) → NaHSO3(aq),
When the alkali is in excess, a sulphite is formed
2NaOH(aq) + SO2(g) →Na2SO3(aq) + H2O(l)
- Reducing property.
- Sulphur dioxide reduces dichromate ions into chromium (III) Ions. This is the test for Sulphur dioxide
CrO72- (aq) + 3SO2(g) + 2H+(aq) →2Cr3+(aq) +3SO42- + H2O(l)
- SO2 reduces acidified purple manganite (VII) ions, MnO4 to Colorless manganese (II) ion, (Mn2+). SO2 Itself being oxidized to sulphuric (VI) acid.
2MnO-4(aq)+5S02(g) +2H2O(l) →2Mn2+ (aq)+5SO2- +4H+(aq).
- Bleaching property.
Sulphurous acid bleaches by reduction, in which it supplies oxygen to the dye.
SO2(g) + H20(l)→H2SO3(aq)
H2SO3(aq) + ( dye +O) → H2SO4(aq) + dye- colorless
- Oxidizing property.
- Sulphur dioxide oxidizes hydrogen sulphideinto Sulphur and water.
- SO2 + 2H2S(g) →3S(s) + 2H2O (l)
- A yellow deposit of Sulphur is formed.
- It oxidizes magnesium into magnesium oxide and is itself reduced into Sulphur.
Mg(s) + SO2(g) →2MgO(s) + S(s)
Uses of sulphur IV Oxide.
- For fumigation to kill insect pest because the gas is poisonous
- For leaching straws, sponges, paper and silk
- Preservation of fruit juices and other food stuffs
- Used as intermediate compound in manufacture of sulphuric acid in contact process
Effects of sulphuric acid
Accumulation of sulphuric acid in the atmosphere can lead to;
- Formation of acid rain which affects animals and plants
- Irritation of throat and eyes due to exposure, if inhaled, it can cause infection of respiratory system
- Acid rain interferes with the PH of soil thus affecting plant growth.
SUMMARY
- Sulphur is a non metal in group (IV) of periodic table it is found in Lousiana and Texas in USA in free from and also in force from and also in performance genes eq at, france are hydrogen sulphide
- Sulphur is extracted by frasch process
- Sulphur occur in two allotropic forms rhombic with octahedral shape and stable above 960C and monoclinic sulphur which is stable above960C it is prismatic.
- Sulphur is yellow in colour and has a variety of use in manufacture of sulphuric acid, fungicides, bleaching agents etc.
- When heated Sulphur, behaves very intresting changing in viscousing till it boils at 4440C.
- Hydrogen sulphide Is a strong reducing agent
- Sulphur has two oxide, Sulphurdioxide and Trioxide. Sulphur dioxide is prepared by reactions of Conc. Sulphuric acid on copper.
- Conc.sulphuric acid is an oxidizing agent dehydrating agent and hydroscope
- The acid can displace more volatile acid from their salts
- Sulphur (IV) oxide has pollution effect on the environment.
END OF TOPIC QUESTIONS
- Name two sources of sulphur
- Explain how frasch process works in extracting sulphur
- Name two allotropes of Sulphur
- Briefly explain what happens when Sulphur is heated till foils. Draw a graph showing the changes of discoursing as temperature increase.
- Jane is given two solutions, suspected to be Sulphite and Sulphates. Describes how she can differentiate the two
- Explain why
- During contact process Sulphur Trioxide is dissolved in sulphuric acid and not water
- A temperature of 4500C is used in contact process although the process require low temperature?
- You should not add water to acid sulphuric acid.
- The reaction between sulphurdioxiede and nitric acid is as follows
SO2(g) + 2HNO3(g) → H2SO4(g) + 2NO2(g)
Identify
Reducing agent
Oxidizing agent
Between SO2and HNO3 Which is strong reducing agent.
- The diagram below illustrates the extraction of sulphur in the Frasch process.
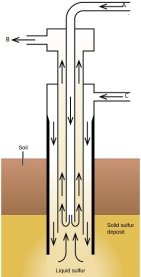
Name the pipe through which heated water is pumped.
- The equation below shows how sulphur (IV) oxide is converted to sulphur (VI) oxide.
2SO2(g) + O2(g) → 2SO3(g) (∆H = -196Kj)
- Name one catalyst used for this reaction
- State and explain the effect on the yield of sulphur (VI) oxide when:
- The temperature is increased
- The volume of oxygen used in increased.
- Explain what happen when,
- Acidified potassium dichromate (vi) is reacted with sulphuric (iv) oxide wite equation for reaction.
- Concentrated sulphuric acid is poted in sugar on bench
- Stating from sulphur, describe how you can prepare
- Plastic sulphur
- Sulphur dioxide
- Hydrogen sulphide
- Hydrogen sulphide
- Complete this equations
H2S(g) + O2 → ?
H2S(g) + O2→?
- Describe an experiment which can be used to show that Sulphuric acid is hygroscopic
- Explain why paper turn brown after being exposed to sunlight for long time.
b. how do bleaching agent of chlorine and Sulphur dioxide differ?
c. which is the best bleaching agent.
11. Explain two ways which can be used to show a gas is Sulphur (IV) Oxide
- When a sample of concentrated sulphuric acid is left in an open beaker in the laboratory for two days, its volume is found to have increased.
- State the property of the concentrated acid that is shown by this observation
- State one use of concentrated sulphuric acid that depends on the property in a above
- Using an equation explain how sulphur (IV) oxide bleaches substances.
- Name the catalyst preferred in the contact process of manufacturing sulphuric acid and explain why it is preferred.
QUESTIONS AND ANSWER SECTION.
2. study the flow chart below and answer questions that follows;
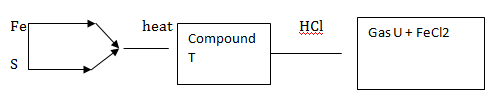
- Name T and U
- Give the chemical test to identify gas U
II. i) List down different types of amorphous
ii) State three other uses of sulphur in industries.
Solution.
T- FeS
U- H2S
b) Hydrogen sulphide turns moist lead II Acetate paper black forming lead II Sulphide
(CH3COO)2Pb(s) + H2S(g) →2HOOCH3(aq) + PbS(s)
II. i) – Plastic sulphur
- Colloidal sulphur.
ii) uses of sulphur
- Manufacture of sulphuric acid
- Manufacture of chemicals such carbon disulphide
- Manufacture of matches and gun powder
3. explain what happens when sulphur is heated.
Solution.
When solid sulphur is heated, it melts and becomes mobile then viscous then mobile again on futher heating.
The sulphur S8 molecules becomes individuals becomes sulphur molecule during melting making liquid mobile. The mobile break up open, join to form long chains which get entangle making the liquid viscous. The chain break-up and straighten out. The molecule break into individual atoms during boiling, making the liquid mobile again.
4. What changes would you observe when powdered roll of sulphur is heated in a test tube just to below its boiling point?
Solution;
Sulphur melts at 113oc to give a mobile amber liquid. As the temperature rises, the liquid darkens and at about 1800C, IT BECOMES VERY viscous(thick) . if the temperature is increased, further, the liquid once more becomes mobile.
5. The flow chart below shows how sulphuric acid is prepared;
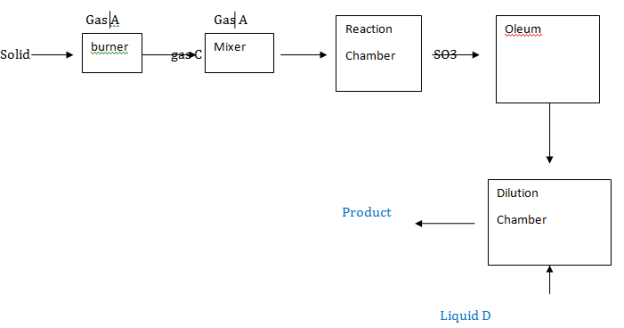
(a) Identify gas A, B, C liquid D and Substance E
(b) What catalyst is used in reaction chamber
© write an equation for reaction at chamber
(d) what would happen if concentrated sulphuric acid is added to;
i) sugar
ii) copper II sulphate
(e) state the uses of sulphuric acid.
Solution.
(a)
- Solid B – Sulphur
- Gas C- Sulphur dioxide
- Liquid D- Water
- Substance E- H2SO4
- Gas A- Oxygen
(b) Vanadium V Oxide.
©2SO2(g)+O2(g) ⥧2SO3(g)
(d) Cane sugar- the sugar will get dehydrated and black carbon remains, smell of sulphur dioxide is also noticed. The elements hydrogen and oxygene are removed by the concentrated sulphuric acid.
![]() C12H22O11-11H2O12C + Heat…
C12H22O11-11H2O12C + Heat…
Heat is generated, and thus reduce some sulphuric acid to sulphur dioxde.
Copper II Sulphate- the blue crystals of copper II Sulphate gradually turn white as they become anhydrous loosing water of crystallization.
![]() CuSO4.5H2OHEATCuSO4+ 5H2O.
CuSO4.5H2OHEATCuSO4+ 5H2O.
(e)
- Lead acid batteries
- Manufacture of fertilizer
- Making detergent and soap
- Used in pickling steel.
6. Sulphuric acid is manufactured by contact process,
(a) State factors to consider when setting up and industry
(b) name raw material required
© state condition is contact process.
(d) (i) How is concentrated sulphur acid is prepared?
ii) explain how unchanged sulphur is removed from the system.
Solution.
(a)
- Availability of raw materials
- Availability of good transport network
- Environmental factors
- Availability of market.
(b) Oxygen and sulphur
© catalyst- vanadium V oxide
- Temperature of 400-5000c
![]() (d) (i) – By diluting oleum with water;H2S207(l) + H2O(l) 2H2SO4(l)
(d) (i) – By diluting oleum with water;H2S207(l) + H2O(l) 2H2SO4(l)
(ii) By absorbing it using calcium hydroxide solution..
7. The set-up below is used to prepare sulphur trioxide
(a) Name gas, N, M, catalyst used and solid Y
(b) Why is it necessary to use a drying agent in solid Y
© name a suitable drying agent to dry gas M and N
(d) How can you test for sulphur dioxide gas.
Solution.
(a)
- Sulphur dioxide
- Oxygen
- Vanadium V Oxide
- Solid Y, Anhydrous calcium chloride.
(b) to prevent atmospheric moisture from coming into contact with sulphur trioxide since moisture would combine with it forming sulphuric acid.
H2O(l)+SO3(g) →H2SO4(l)
© Conc. Sulphuric acid.
8. Why is concentrated sulphuric acid used as a drying agent?
Solution.
Because it is hygroscopic and dehydrating property makes it suitable to be used as a drying agent.
SAMPLE NECTA QUESTIONS.
2015
- (a) Briefly explain what will happen when:
- concentrated sulphuric acid is exposed to the atmosphere.
- iron (II) sulphate is exposed to air for a long time.
- a bottle containing AgNO3 is left open.
(b) Give three applications of the process of neutralization in daily life.
CARBON
Carbon exists mostly in free state as graphite and diamond. It exists in a number of forms such as wood charcoal, animal charcoal, coke and soot or lamb black. It occurs in many substances eg, carbon dioxide, carbonate, shells, and in all organic matter.
Allotropes of carbon.
Allotropy is the existence of an element in more than one form in the same physical state.
Allotropes- are different forms of an element with different physical properties but the same chemical properties.
Allotropes of carbon.
- Diamond
- Graphite
- Amorphous
Diamond and graphite are crystallized forms while amorphous is non-crystallized form.
Diamond
Diamond is the hardest substance known on earth. In diamond, each carbon is joined by covalent bond to four other carbon atoms. This forms a tetrahedral structure. The forces that bond the atoms are equally strong in all directions in diamond. Compared to graphite, diamond is more denser because its atoms are closely packed. Diamond has no free electrons and hence it is an insulator meaning it does not conduct electricity.
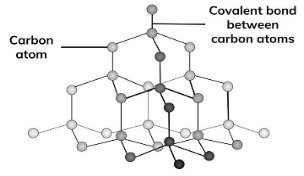 Figure 1.12. Diamond structure
Figure 1.12. Diamond structure
Physical properties of diamond.
- Has high melting and boiling point due to strong covalent bonds.
- It is a bad conductor of heat and electricity
- It is colourless, transparent and sparkling
Uses of diamond.
- Because of high refractive index, it is used as a gemstone or valuable stone
- For cutting glass, drilling of rocks, and for abrasive purposes because of its hardness
- Used to make jewellery, such as rings, because of its shiny and attractive appearance
Graphite.
In graphite, only three out of four electrons in carbon are used in bonding. One electron is thus left to move free. For this reason, graphite is able to conduct electricity
The arrangement of atoms in graphite forms hexagonal rings of six carbon atoms. The hexagonal layers are joined by weak covalent bonds-allowing them to slide over each other.
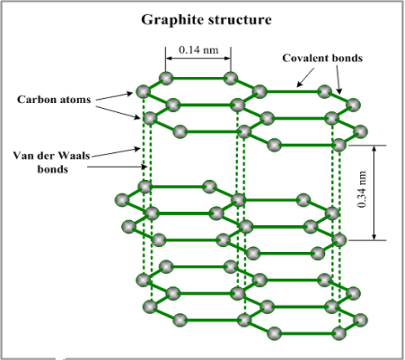
Figure 1.13. Graphite structure
Physical properties of graphite.
- Due to delocalized electrons, it is a good conductor of electricity
- It is soft and slippery because of weak van der waal forces
- Has high melting and boiling point because of presence of strong electrovalent bonds.
Uses Of Graphite.
- It is mixed with clay to make lead pencils.
- Used as an electrode in electrolytic cell because it is very innert
- Used as a lubricant because of its greasy and slippery property
Amorphous carbon.
Is a non crystallined form of carbon. The arrangement of atoms are disorderly. This allotrope does not occur naturally but exists in various forms.
- Charcoal
- Coke
- Lampblack
- Charcoal.
Charcoal exists in three main forms, animal charcoal, suagar charcoal and wood charcoal.
Animal charcoal
Formed when bones are heated in limited supply of air. It is used to whiten crude sugar by removing the brown impurities.
Sugar charcoal
It is formed by destructive distillation of sugarcane. Also formed when conc. Sulphuric acid dehydrates sugar. This charcoal is used to absorb gases in chemical processes.
Wood charcoal
Formed by destructive distillation of wood. It is used as gas masks because it absorbs poisonous gases
- Coke.
Is formed when coal is heated in absence of air.
Uses of coke.
- Used as a fuel in furnaces, boilers and ovens
- It is used as a reducing agent in the extraction of metals
- Used as a source of gaseous fuel
- Lamb black.
They are formed when petroleum, kerosene, turpentine, and natural gas and other hydro-carbons are burnt in limited supply of air.
Uses of lamb black.
- Making Indian ink, black shoe polish, carbon paper, and printers ink.
- In making rubber tyres, which makes the rubber strong.
Reducing properties of carbon.
Carbon is a reducing agent.
- It reduces the oxides of metals to their respective metals and carbon dioxide
Lead (II) Oxide + Carbon→ lead + carbon dioxide
Copper(II) Oxide + Carbon→copper + Carbon IV Oxide.
- Carbon also reduces hot nitric V acid to nitrogen (IV) oxide (NO2), and hot suphuric acid to Sulphur IV acid respectively.
Carbon + Sulphuric Acid→carbon dioxide + Sulphur dioxide + water
Carbon + nitric acid→carbon dioxide + nitrogen IV Oxide + Water
Carbon (IV) Oxide.
Occurrence.
It occurs in the atmosphere where it occupies 0.03%. it is also a constituent of bodies of plants and animals.
Preparations.
It is prepared by reacting dilute hydrochloric acid with marble chips- calcium carbonate.
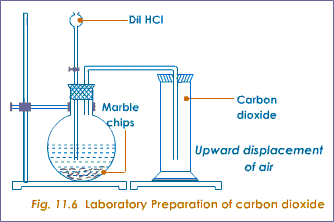
Figure 1.13. Preparation of carbon dioxide
The dilute hydrochloric acid reacts with the carbonate forming effervescence with evolution of carbon dioxide.
Calcium carbonate + hydrochloric acid →calcium chloride + carbon (V) Oxide
CaCO3(s) + 2HCl(aq) →CaCl2(aq) + C02(g)+ H20 (l)
Physical properties of carbon dioxide.
- It is colourless, odourless gas with a faint acid taste
- It is soluble in water forming carbonic acid
- It is heavier, than air.
Chemical properties.
- The gas does not support combustion
- Dissolves in water forming weak carbonic acid
- It reacts with alkalis to form carbonates
- Carbon dioxide can react with burning magnesium. When burning magnesium is introduced in a gas jar fully of carbon dioxide, it continues to burn oxidizing the magnesium into magnesium oxide while itself is reduced to carbon. The heat produced by burning magnesium decomposes carbon IV Oxide to carbon and oxygen.
The oxygen reacts with magnesium.
Uses of carbon (IV) Oxide
- Used as fire extinguisher because it does not support combustion
- Use to make fizzy drinks
- Used as a refrigerant
- Used in baking bread and coke.
- In fruit preservation, by creating an atmosphere in which there is less oxygen and more carbon dioxide.
Test for carbon dioxide
This gas is tested by passing through lime water in which the lime water changes into white precipitate.
The formation of white precipitate is due to the presence of solid calcium carbonate
Ca(OH)2aq + CO2(g) →CaCO3(s) + H2O (l)
When excess carbon dioxide is passed, the solution turns into colourless due to formation of soluble calcium hydrogen carbonate.
QUESTION AND ANSWER SECTION.
1.
- Define allotropy
- Name allotropes of carbon
- Define amorphous carbon and give examples
- State the uses of amorphous carbon
Solution.
- Allotropy is the existence of an element in more than one physical form.
- – diamond
- Graphite.
c) amorphous carbon is a non-crystallined form of carbon which consists of minute fragements of graphite.
Examples, sugar charcoal, wood charcoal, animal charcoal, lamb black, sooty and coke.
d) Wood charcoal- Source of energy
- Used to absorb bad odour in urinals
Animals charcoal- Used to remove brown colour from crude sugar.
Lamb black- Making shoe polish, carbon paper, printers ink tyres
Coke- Used in blast furnace, ovens, and bottles
- Used as reducing agent in metal extraction.
2. Give reasons why;
- Graphite can conduct electricity while diamond cannot
- Graphite is soft but diamond is hard
- Graphite has lower density than diamond
- Both graphite and diamond have high melting points
Solution.
- In diamond each carbon uses its four bonding electron to form covalent with other atoms. In graphite, each carbon atom is bonded to three other even though four electrons are present. The extra electron is free to move around the structure, making it possible to conduct electricity. This is called delocalized electrons.
- Graphite has layer off hexagon of carbon atoms. Bonding between carbon is very strong but the layer are held by weak vander waal forces. This makes the layer slide on each other making graphite soft. All bonds in diamond are therefore very strong.
- Graphite has a more open structure than diamond hence has a low density
- Because they consists of giant structures of atoms.
4. Explain four fuels of carbon
Solution.
i) Wood- it occurs naturally and contains 50% carbon
ii) coal gas- obtained from the destructive distillation of coal. It consists of methane, carbon monoxide and hydrogen.
iii) Producer gas- Formed by passing air over rd hot coke in furnace. The reaction is exothermic
C(s) + O2(g)→CO2(g)
CO2(g)+ C(s)→ 2CO(g)
It contains 1/3 CO and the rest nitrogen from the air.
iv) Water gas- Is produced by passing steam over heated white hot coke above 10000C in a furnace.
5. Describe preparation and collection of carbon dioxide in laboratory.
Solution.
Carbon dioxide is prepared by reaction of dilute hydrochloric acid on marble chips. The gas given off is passed over water to remove sprays(HCl) and is then collected by downward delivery as shown below.
The equation for the reaction is;
![]() CaCO3(s) +2HCl(aq) CaCl2(aq) + CO2(g) + H20(l)
CaCO3(s) +2HCl(aq) CaCl2(aq) + CO2(g) + H20(l)
6. (a) mention two ways in which reaction between marble chips and HCl can be increased.
(b) explain what happens when C02 gas is passed through lime water in few minutes, then several minutes, use an equation.
© give reasons why it is not advisable to use sulphuric acid instead of HCl.
Solution.
(a)
- Using a more concentrated acid
- Using small marble chips
- Increasing temperature
(b) lime water will iniatialy turn milky, few minutes if allowed. However, on passing for several minutes, lime water will change into colourless solution. Initialy white ppt is formed;
CO2(g) + Ca(OH)2(aq)→ CaCO3(s)+H20(l).
On passing for several minutes, white precipitate disappears forming a clear solution.
CaCO3(s)+H20(l)+CO2(g) →Ca(HCO3)2(aq)
© With calcium carbonate, sulphuric acid forms calcium sulphate which is insoluble. This forms a layer around marble chips preventing further reaction.
7.(a) mention the uses of carbondioxide
(b) mention two ways in which carbon dioxide is added into the air and how it is removed.
Solution.
(a)
- Used as a fire extinguisher
- Used in aerated or fizzy drinks
- Used in refrigeration to keep ice cream cold
- Making of baking powder
- Manufacture of sodium carbonate.
(b) carbon dioxide is added into air by;
- combustion of substances rich in carbon
-respirations
Carbon dioxide is removed by;
- Photosynthesis
- Dissolving the gas in water
8. Give an explanation for the following;
i) dry ice is a better refrigerant than ordinary ice
ii) when lighted splint is plunged into gas jar of CO2 it goes off.
iii) when a piece of burning magnesium is lowered into gas jar of carbon dioxide, it continues to burn
(b) state the uses of diamond and graphite
Solution.
- Because dry ice solid sublimes when warmed leaving no residue
- Carbon dioxide does not support combustion hence it extinguishes it
- Magnesium has high affinity of oxygen. It combines with it to form MgO a white solid, and carbon black is deposited
Mg(s) +CO2(g) →MgO(s)+ C(s).
b) Uses of the following;
i) diamond- used as a gemstone owning to its sparkling nature
- making drilling bits due to its hardness
ii) graphite- Used as a lubricant due to its slippery nature
- Used as regulator in atomic reactors
- Used as electrodes in battery cells
9.(a) How is carbon dioxide obtained industrially?
(b) explain what happens when metal carbonates are heated.
Solution.
(a) On large scale carbon dioxide is obtained by;
- Burning coke in air
- as a by-product in ethanol preparation
(b) Insoluble carbonates decompose to form metal oxide and carbon dioxide
- CaCO3→CaO(s) + CO2(g)
- ZnCO3(s)→ZnO(s)+CO2(g)
CHAPTER SUMMARY
NON METALS AND THEIR COMPOUNDS
- Non metals react by gaining electrons; they are electronegative.
- Electro negativity is the tendency of a non metal to gain electrons.
- Non metals displace each other in their aqueous compounds.
- Chlorine bleaches only in the presence of water.
- Chlorine reacts by gaining electrons. it is an oxidizing agent.
- Chlorine gas is a poisonous. It is prepared in a fume cupboard.
- Hydrogen gas is poisonous. It is prepared by the action of concentrated sulphuric (IV) acid on rock salt.
- Hydrogen chloride gas turns wet blue litmus paper red; it has acid properties in solution form.
- Hydrogen chloride gas forms white fumes of ammonia chloride with ammonia gas. This is the test for hydrogen chloride gas
- Sulphur is extracted from natural deposits by the frasch process.
- The main allotropes of sulphur are rhombic, monoclic and plastic sulphur.
- Sulphur exhibits both oxidizing and reducing properties.
- Sulphuric (VI)Acid is manufactured by the contact process.
- Concentrated sulphuric acid (VI) acid is adehyrating agent. It can remove water or elements of water from compounds.
- Sulphur (IV) oxide causesbleaching by reaction.
- Sulphur (IV) oxide reacts as an oxidizing agent with some reagents during which it reduces to sulphur.
- Sulphur (IV) oxide is used as an intermediate in the manufacture of sulphuric (IV) acid.
- Nitrogen gas can be prepared in the laboratory by isolution from air.
- Nitrogen is inert at room temperature due to the strong triple convalent bonds between its atoms.
- Nitrogen is a major raw material in the manufacture of ammonia gas.
- Ammonia is prepared in the laboratory by heating an ammonia salt with a base.
- Ammonia is highly soluble in water and exhibits alkaline properties in solution form,
- Ammonia forms white fumes of ammonium chloride when reacted with hydrogen gas. This is the test for ammonia.
- Carbonate (IV) oxide can be prepared in the laboratory by the action of dilute acids on suitable carbonate.
- Carbonate (IV) oxide forms a white precipitate with calcium hydroxide or barium hydroxide. This is the test for the carbon (IV) Oxide.
- Allotropy is the existence of an element in more than on form but in the same physical state.
NON METALS AND THEIR COMPOUNDS
- Define allotropy.
- Name two crystalline allow tropes of carbon.
- Damp blue and red litmus paper loses their color when put in a jar of chlorine. By use of equations explain.
- State one major industrial use of hydrogen chloride gas.
- State the differences between rhombic and monoclicsulphur.
- Explain what is observed when concentrated sulphuric (IV) Acid is added to :
- Sugar
- Hydrated copper (II) sulphate
- By the use of relevant chemical equations explain how acid rain is formed.
- State and explain any two uses of nitrogen.
- Describe the chemical test for ammonia.
TOPICAL EXAMINATIONS
CHEMISTRY FORM FOUR
NON-METALS
SECTION A 20 MARKS.
- The method of collecting hydrogen chloride gas in a class experiment is known as:
- Downward displacement of water
- Downward displacement of air
- Upward displacement of air
- Fountain
- Condensation
- Which action should be taken immediately after concentrated sulphuric acid is spilled on the skin?
- It should be rinsed off with large quantities of running water.
- It should be neutralized with solid CaCO3.
- It should be neutralized with concentrated NaOH.
- The affected area should be wrapped tightly and shown to medical health provider.
- It should be neutralized with concentrated KOH.
- The only metal which does not react with dilute hydrochloric acid is:-
- Magnesium
- Aluminium
- Copper
- Zinc
- Sodium
- Which among the following equations correctly shows the reaction between chlorine gas and water?
- C l2(g) + H20(1)→CI2(g)
B2C12(g) + 2H20(1)→ 4C1-1(aq) + 02(g) + 2H2(s)
- Cl2(g) + H20(1)→HCl + HOCI(aq)
- 2Cl2(g) + 2H20(I) →2H0C1 +H2(g)
- 2C12(g)+ 3H200) → C12 (.0 + 2H30+
(v) Which of the following pair of gas can be prepared in the laboratory an: collected over water?
- Oxygen and Ammonia
- Hydrogen and Hydrochloric acid
- Hydrogen and Oxygen
- Oxygen and Hydrogen chloride
- Hydrogen and Ammonia
(vi) Two substances are allotropes of carbon if they:
A. both reduce heated iron (III) oxide to iron
B. have different crystalline structure
D. have equal masses
C. have equal shape
E. have the same arrangement of atoms
vii)Which of the following sets of elements is arranged in order of increasing electro negativity?
A. Chlorine, fluorine, nitrogen, oxygen, carbon
B. Fluorine, chlorine, oxygen, nitrogen, carbon
- Carbon, nitrogen, oxygen, chlorine, fluorine
- Nitrogen, oxygen, carbon, fluorine, chlorine
- Fluorine, nitrogen, oxygen, chlorine, carbon
viii) The gas formed when dilute nitric acid reacts with magnesium metal is;
- Nitrogen
- Hydrogen
- Oxygen
- Nitrogen dioxide
ix) which of the following is true about carbon?
- Carbon dioxide is very acidic
- Solid carbon d
2. Match the items in list A with the responses in list B by writing the letter of the correct response beside the item number.
| List A | List B |
|
0. Characteristic yellow flame.
|
3.(a) With the help of chemical equation, what will be observed when ammonia reacts with:
- Hydrogen chloride?
- Copper (II) oxide?
(b) It is not advisable to sleep inside a house which is not well ventilated with a burning wooden charcoal. Give a reason for that and write the chemical equation to represent your answer.
4 .(a) The chemical properties of concentrated sulphuric acid can be grouped into oxidizing property and dehydrating property. In which property should sulphuric acid be grouped when it reacts with copper metal? Give reason and write the equation of the reaction.
(b)The preparation of chlorine gas can be represented by the following equation:
Mn02 + 4HCI→ MnCl2 + 2H20 + Cl2.
Calculate the number of moles of HCI which are needed to react with 20g of Mn02 and list two main chemical properties of chlorine gas.
5. (a) (i) What is the name given to the different forms of the element which exists in the same physical state?
(ii) Carbon exists in two different forms of the same physical state and one of those carbon forms is represented by structure X below. Give the name of the carbon form with structure X.
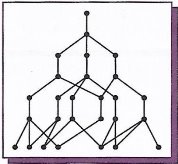
- Name the second form of carbon.
- State one property and one use which depends on the property you have stated for each form of carbon.
- Carbon can be used to convert copper II oxide to copper as shown in the equation.
C(S) + 2CuO(s) → 2Cucs) +CO2(g)
What is the function of carbon in this equation?
- Calculate the mass of CuO which can react with 12g of carbon in the equation given in 5 (b) (i) above.
- What is the effect of carbon monoxide in the blood?
7.(a) Identify the substances by using the following information:
- A solid is yellow when hot and white when cold.
- When water is added to a white powder heat is evolved and the white powder changes to blue crystals.
- An aqueous solution of a greenish crystalline sulphate forms a pale-green precipitate with sodium hydroxide solution which turns to brown on standing and when exposed to air.
- A colourless gas turns a yellow acidified potassium dichromate paper to green.
- A colourless gas becomes brown on exposure to air.
(b) With the help of chemical equations explain what happens to the following compounds of ammonia when heated in separate test tubes:
- A mixture of ammonia chloride and sodium hydroxide solution
- Ammonium chloride crystals
- Ammonium nitrate crystals
- Ammonium sulphate crystals
- Ammonium nitrite crystals
8. The Diagram below shows the preparation of chlorine gas in a laboratory fume-chamber. Study the diagram and answer the questions that follow.
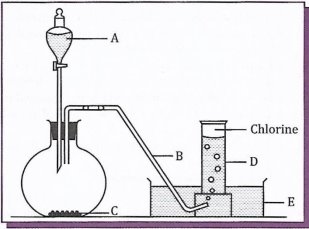
(a) What do letters A, 8, C, D and E represent?
- Why is the gas prepared in the fume-chamber?
- Can the gas be collected over water? Why?
- What will happen to a damp blue litmus paper if it is introduced into a gas jar full of chlorine gas?
- What will happen if a gas jar of hydrogen sulphide is inverted over a gas jar of chlorine such that the two gases get mixed? Write a balanced equation for the reaction which will take place between hydrogen sulphide gas and chlorine gas.
(b) (i) List down two uses of chlorine gas.
(ii) Give a balanced chemical equation for the method of preparation of chlorine used in this question.
9. (a) Write ionic equations for the following:
- Laboratory preparation of ammonia gas.
- Precipitation of barium sulphate from barium chloride and sodium sulphate and silver chloride from a soluble chloride.
- Neutralization of a strong acid and a strong alkali.
(b) Consider the following elements of group seven in the order in which they appear in their group in the Periodic Table. F, C, Br, and I.
- Which element is the most electronegative?
- Name the least electronegative element.
- Which element has the largest atom?
- Write the electronic configuration of the chlorine atom.
(c) Define electro-negativity
11. Which method is used in the laboratory gas preparation of:
- Ammonia?
- Chlorine?
- Hydrogen?
Give reasons for your answers.
(c) What will happen when:
- yellow flowers are introduced into a gas containing chlorine gas?
- a burning splint is introduced into a gas jar containing hydrogen gas?
- a glass rod which was dipped in concentrated hydrochloric acid is introduced into a gas jar containing ammonia gas?
- sulphur dioxide gas is bubbled through a yellow acidified potassium dichromate solution?
12. The preparation of ammonia in the laboratory is done by heating a mixture of ammonium chloride and sodium hydroxide.
(a) (i) Write a balanced chemical equation for the above reaction.
(ii) Using balanced chemical equations, state how ammonia reacts with hydrogen chloride gas and heated copper (II) oxide.
(b) (1) State two uses of ammonia.
(ii) Name the catalyst used in the preparation of ammonia.
(c) Explain each of the following reactions, giving observations and equations.
- Aqueous ammonia is added to iron (III) chloride, little by little, until in excess.
- Sodium nitrate is strongly heated.
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






