CHAPTER 1
1.0 CHEMICAL EQUATIONS
1.10 KEYTERMS AND CONCEPTS.
- A chemical equation- this is a representation of chemical chemical change by use of symbols and formula.
- Word equation-This is an equation written using chemical names
- Molecular equation- this is an equation written using chemical formula
- Ionic equation- this is writing equation using only ions that take place in chemical equation.
- Decomposition- the splitting up of a substance often on heating
- Synthesis reaction- is a type of chemical reaction in which elements combines to form a compound.
- Displacement reaction- this is a type of reaction in which a more reactive element displaces a less reactive element in a compound.
- Precipitation reaction- is a type of reaction which results into formation of a solid when two solution are mixed.
- Stoichiometric equation- is a balanced chemical equation written with all state symbols.
- Spectator ions- these are ions which do not take part in chemical reaction
How To Tackle Questions On This Chapter.
- The knowledge of chemical equation very important because almostall topics in chemistry will require you to write equation.
- A grasp of chemical symbols of element, symbols of common radicals and in some cases complex compounds is very important.
- A student will also need to understand how to assign oxidation state and valances to elements and group of atoms (radicals).
- Understandstate symbols of various substances
- Understand the solubility of various substances in water before writing chemical equation.
- When writing any chemical equation, ensure that it is well balanced and has all state symbols.
- Write a word equation, then molecular equation and finally ionic equation.
Introduction.
Chemistry deals with the study of properties of substances and the interaction between different substances. During our daily life, different changes takes place in nature; for example a banana fruit ripens naturally, food get cooked when heated, iron get rust when exposed to air and milk gets sour when left for a long time. These interactions are referred to as chemical reactions. Chemical reactions occurs when change occurs, converting to one or more other substances. The substances undergoing the newly formed substances are known as products. In form two you learnt about reversible and irreversible changes. Reversible changes are also called physical changes while irreversible changes are called chemical changes. The following are characteristics of a chemical change
- There can be observable change in colour
- New products can be formed during chemical reaction
- There is absorption or evolution of heat
- Theproductformed during a chemical change do notback to reactants once conditions are reversed.
- There might be change in mass or evolution of a gas
All chemical reactions are expressedby chemical equations or by ionic equations, whereby one or more substances which can be elements undergo a chemical change to form a product.In this topic you will learn about how to use chemical symbols and formulate to describe a chemical reaction.
1.2 Chemical equations
A chemical equation is an equation which represents a chemical change by means of symbols and formulae.
Any chemical equation has two main parts namely reactants and products. The reactants are the substancethat interact in a reaction and the products are the new substance formed during the reaction.
The reactants are actually separated from reactants to the left hand side and products to the right side of the arrow. (→) means to form the products shown. i.e
A + B →C + D
(Reactants)(Products)
The arrow can also mean decomposes to form the products shown i.e.
AB → A + B
The (+)sign in an equation means reacts with when on the left of the arrow. While on the right hand side it means and.
The symbol + on the left hand side of the equation means, reacts with and on the right hand side it means “and” while the arrow means to form
An equation such as this Q + P → S + T can be interpreted as Q reacts with P to form S and T.
Examples of chemical reactions;
Sodium + Oxygen →Sodium Oxide
Na(s)+ O2(g) →Na2O.
Hydrogen+ Oxygen →Water
H2(g) + O2(g) →H2O(l)
Regarding chemical equations,
1.3 Types of chemical reactions
The four types of chemical equations are:
- Decomposition reaction.
- Combination or synthesis reactions
- Displacement reactions
- Double decomposition reaction
- Decomposition reaction
Decomposition reaction are those in which compounds break down into simpler substances when heated,
AB ![]() A + B
A + B
Calcium carbonate ![]() calcium oxide + carbon dioxide
calcium oxide + carbon dioxide
These are two types of decomposition reactions.
- Thermal decomposition
- Thermal dissociation
- Thermal decomposed
Thermal decomposition is the decomposition of a compound by heat into simpler substance which do not recombine on cooling.
The reaction can be represented as follow:
AB → A + B
For example;
When potassium chlorate (V) is heated, it decomposes into potassium chloride and oxygen gas.
Potassium chlorate (VI) → Potassium chloride+ oxygen gas
KClO3 ![]() KCl + O2(g)
KCl + O2(g)
- Thermal dissociation
Thermal dissociation is the decomposition of a compound by heat into simpler substances which recombine on cooling to form the original compound.
The reaction can be represented by follows.
AB → A + B
For example
Ammonium chloride dissociates on heating into ammonia and hydrogen chloride, which recombine on cooling.
Ammonium chloride → Ammonia gas + Hydrogen Chloride
Thermal dissociation is an example of a reversible reaction.
A reversible reaction is one which can proceed in either direction, depending on the condition s such as temperature and pressure under which it is carried out.
Examples of reversible reactions are:
- Hydrogen + oxygen ↔ Water
2H2 (g) + O(g) ↔water
- Sulphur (IV) oxide +↔ Sulphur (VI) Oxide
2SO2(g) + O2(S) ↔2SO3(g)
- Ammonia chloride ↔ammonia + hydrogen chloride
NH4 Cl(s) ↔NH3(g) + HCl(g)
- Calcium carbonate ↔Calcium oxide + carbonate (IV) oxide
CaCO3(s) →CaO(s) + CO2(g)
II) Combination or synthesis reactions
Combination or synthesis reactions are those in which elements combine to form a single compound.
The reaction can be represented as follows,
A + B →AB
Forthe example,
Under special conditions, ammonia gas can be synthesized from its elements nitrogen and hydrogen.
Nitrogen + hydrogen →ammonia + heat
N2 (g) + 3H2(g) + heat
III) Displacement reactions
Displacement reactions are reactions in which an element displaces another element from a compound.
The reaction can be represented as follows:
A + BC → AC + B
In this reaction, A displaces B from BC to form AC.
For example
When chlorine gas is bubbled through potassium iodide solution, it displaces iodine to form a reddish- brown solid i.e iodine to form a reddish brown solid i.e iodine and a potassium chloride.
Chlorine + potassium iodide →Potassium Chloride + Iodine
Cl2(g) + 2KI(aq) → 2KCl + I2 (s)
Colorlessreddish – brown
IV) Double decomposition reactions
Double decomposition reactions are reactions in which two soluble compounds decompose and then exchange their radicals to form two compounds in which one must be a solid. This type of reaction is also known as precipitation reaction.
The reaction can be represented as follows:
- AB(aq) + CD(aq) →AD(s) + CB(aq)
Thereactionbetween silver nitrate solution and sodium chloride solution. A solid silver chloride is formed as a white precipitate.
Silver nitrate + sodium chloride → is formed as a white precipitate.
Silver nitrate + sodium nitrate
AgNO3 (aq) + NaCl(aq)→ AgCl(s) + NaNO3(aq)
(b) Steps to follow when writing chemical equations
- Find out the names of all the reactions and products.
- Write the equation in words.
- Write the equation using the correct symbols and formulae.
- Balance the equation. That is make the number and kind of atoms equal on both sides of the equation.
- Introduce state symbols. i.e g for gas, s for solid and I and I for liquid.
(c) Balancing chemical equations
Balancing an equation is the process of making the number of each kind of atoms equal on both sides of the equation.
For example,
The equation for the reaction between hydrogen and oxygen seems to be:
H2 (g) + O2(g) → H2O(I)
This is incorrect, because there are two (2) Atoms of oxygen on the left hand side but only one (I) on the right. We can make the number of oxygen atoms equal writing the equation as follows:
H2 + ![]() O2 →H2O
O2 →H2O
However, this also is not correct, because half a molecule does not exist. By doubling this equation it becomes correct. Thus
2(H2+ ![]() O2 →H2O) Becomes
O2 →H2O) Becomes
2H2 (g) + O2(g) → 2H2O(I)
For example
- KClO3 →KCL + O2
- KClO3 →KCL +
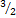 O2
O2 - 2{KClO3}→2{KCL +
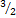 [O2]}
[O2]} - →2KClO3 →2KCL(s) +3O2(g)
- P + O2 → P2O5
- 2P +
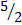 O2 → P2O5
O2 → P2O5 - 2(2P +
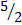 O2 → P2 O5)
O2 → P2 O5)
This gives
4P (s) + 5O2 (S) →2P2O5(s)
Example 1
Sodium reacts with water to form a solution of sodium hydroxide and hydrogen gas. Write the formula equation for the reaction.
Rule 1
Reactants sodium hydroxide and hydrogen
Rule 2
Sodium + water → sodium hydroxide + hydrogen
Rule 3
Na + H2O → NaOH + H2
2Na + 2H2O → 2NaOH + H2
Rule 5
2Na(s) + 2H2O(I) → 2NaOH(aq) + H2(g)
Example 2
Write the formula equation for the reaction that would that would occur during burning of Magnesium in oxygen gas.
Rule 1
Reactants – sodium peroxide and water
Products sodium hydroxide and oxygen gas
Rule 2
Water + sodium peroxide →hydrogen
Rule 3
Na + H2O → NaOH + H2
Rule 4
2Na + 2H2O → 2NaOH + H2
Rule 5
2Na(s) + 2H2O(I) → 2NaOH(aq) + H2(g)
Example 2
Write the formula equation for the reaction that would occur during burning of magnesium in oxygen gas.
1.4 IONIC EQUATIONS
An ionic equation is an equation which shows only the actual ions taking part in a chemical reaction.
- We write ionic equation leaving out the spectator ions.
- Spectator ions are those ions that do not change during a chemical reaction, hence they are found on both sides of the equation.
- They are aqueous before and after the reaction.
- They are aqueous before and after reaction, therefore they are cancelled out when writing ionic equation.
Molecular versus ionic equations
A molecular equation is a balanced chemical equation in which ionic compounds are written as molecules rather than as ions. For example
KNO3 (aq) + HCL (aq) → KCL(aq) + HNO3 (aq)is a molecular equation while
K+(aq) +NO3 –(aq) + H+(aq) + Cl- (aq)→ K+(aq) +NO3 –(aq) + H+(aq) + Cl- (aq) is an ionic equation.
Steps for writing ionic equations
- Write the correct formula equation for the reaction.
- Balance the chemical equation.
- Identify solids, liquids, aqueous solutions and Gaseous.
- Identify soluble ionic compounds and insoluble ionic compounds.
- Split soluble ionic compounds into individual ions
- Cancel out all spectator ions
- Write the net ionic equation using remaining ions.
Write the ionic equation for the following reaction.
Barium chloride + Sodium Sulphate →Barium chloride + Sodium sulphate
Step 1
BaCl2(aq) + NaSO4(aq) → BaSO4(S) + NaCl(aq)
Step 2
BaCl2(aq) + Na2SO4(aq) → BaSO4(S) + 2NaCl(aq)
Step 3
Solids, liquids, aqueous solutions and gaseous
BaCl2(aq) + NaSO4(aq) → BaSO4(S) + 2NaCl (aq)
Step 4
Soluble ionic compounds: BaCl2 ,Na2SO4 , NaCl,
Insoluble ionic compounds: BaSO4
Step 5
Split all soluble ionic compounds into individual ions. Do not split insoluble ionic products into ions.
Ba2+ (aq)2 + 2CL- (aq) + SO42-(aq) → BaSO4(s) + 2Na+(aq) + 2CL- (aq)
(IV) Cancel out spectator ions.
Ba2+ (aq)2 + 2CL- (aq) +2Na+(aq) + SO42-(aq) → BaSO4(s) + 2Na+(aq) + 2CL- (aq)
(vii) Net ionic equation
Ba2+(aq) + SO2+4(aq) → BaSO4(s)
Example 2
Write the net ionic equation for the reaction between dilute hydrochloric acid and aqueous sodium hydroxide solution.
Solution
- Word equation
Hydration acid + sodium hydroxide → sodium chloride + water
- Balanced formula equation
HCL(aq) + NaOH(aq)→ NaCl(aq) + H2O(I)
- Total ionic equation and cancelling out spectator ions.
H+(aq) + Cl-(aq)+ Na+ (aq) + OH- (aq) → Na+(aq) + Cl- (aq) + H2O(I)
Iv. The net ionic equation
H+ (aq) + OH- (aq) → H2O(I)
Example 3
Write the ionic equation for the reaction betweendilute sulphuric (iv) acid and calcium carbonate solution
- Word equation
Sulphuric (IV) acid + calcium carbonate → calcium sulphate + water + carbon (IV) Oxide
- Balanced formula equation
H2SO4(aq) + CaCO3(aq) → CaSO4 + H2O(I) +CO2(g)
- Total ionic equation and cancelling out spectactor ions:
2H+ (aq) + SO4(aq)2-+ Ca2+(aq) + CO32- (aq) → CaSO4 + H2O(I) +CO2(g)
No spectator ions
- Cancel spectator ions:
2H+ (aq) + SO4 (aq)2-+ Ca2+(aq) + CO32- (aq) → CaSO4 + H2O(I) +CO2(g)
Calcium is slightly soluble in water.
Example 4
Write a balanced ionic equation for the reaction between dilute hydrochloric acid and calcium carbonate.
Hydrochloric acid + calcium carbonate → calcium chloride + water + carbon (IV) Oxide
2HCl(aq) + CaCO3 (s)→ CaCl2(aq) + H2O(I) + CO2(g)
Total ionic equation and cancelling out spectator ions:
2H+(aq) + 2Cl-(aq) + Ca2+(aq) + CO32-(s) → Ca2+(aq)+2Cl-(aq) + CO2(g) + H2O(I)
The net ionic equation is
2H+ (aq) + CO32- (s) → H2 O + CO2 (g)
Example 5
Write the ionic equation for the reaction between sodium hydroxide and dilute sulphuric (VI) acid.
Sulphuric (VI)acid + sodium hydroxide → sodium sulphate + water
H2SO4 (aq) + 2NaOH(aq) → Na2SO4(aq) +2H2O(I)
Total ionic equation and cancelling out spectator ions
2H+ (aq) + SO2-4(aq) + 2Na+ (aq) +2OH-(aq) → 2Na+(aq) + SO2-4(aq) + 2H2O(I)
Net ionic equation is
2H+ (aq) +2OH-(aq) → 2H2O (I)
CHEMICAL EQUATIONS CHAPTER SUMMARY.
- A Chemical equation is an equation which represent a chemical change by means of symbols and formulae.
- A word equation is a chemical equation showing only the names of the reactants and products.
- A formula equation is a balanced chemical equation in which ionic compounds are written as molecules rather than as ions. It is also known as molecular equations.
- Balancing chemical equation is the process of making the number of each kind of the atoms equal on both sides of the equation.
- An ionic equation is an equation which shows only the actual ions taking part in a reaction.
- There are four types of chemical reactions namely:
- Decomposition reactions.
- Combination or synthesis reaction.
- Displacement reactions
- Double decomposition precipitations.
1.5 END OF TOPIC QUESTIONS.
- Fe(g) + S(g) → Fe S(g)
What comment can a chemist give on the above chemical equation?
- The state symbols are not well written
- The condition in which the reaction takes place is not indicated
- The symbol “to form” is not well written
- It is well written.
- Which of the following chemical equation is mechanical?
- Cl2(g) + 2Kl(aq) → 2KCl(aq) + l2(g)
- Cu(g) + HCl(aq) + CuCl2(aq) → H2(g)
- NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + N2O(l)
- Ag+(aq) + Cl-(aq) → AgCl(g)
- How can one classify equation iv A above?
- Double ionization
- Displacement
- Neutralization
- Synthesis
- The equation X2+ + 2e-→X, represent:-
- Oxidation reaction
- Reduction reaction
- Displacement reaction
- Synthesis reaction
- Neutralization
- Which of the following equations represent a neutralization reaction?
- AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
- HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
- NO2(g) + SO(g) + H2O(l) → H2SO4(l) + NO(g)
- H2SO4(aq) + 2n(s) → H2(g) + 2nSO4(aq)
- CaCO3(s) → CaO(s) + CO2
- Name the following chemical reaction H2(g) + Cl2(g) → 2HCl(g)
- Displacement
- Decomposition
- Synthesis
- Neutralization
PART II.
- (a) Write balance molecular equations of the following chemical reactions:
- Barium nitrate solution reacted with sodium sulphate solution
- Solution sodium bicarbonate reacted with dilute hydrochloric acid
- Silver ions reacted with sodium chloride solution
- Write ionic equations of 11 (a) above
- Write ionic equations of the following chemical reactions
- Aqueous sodium carbonate reacts with dilute hydrochloric acid
- Silver nitrate solution reacts with dilute magnesium chloride solution
- Zinc metal is put in a solution of copper sulphate
- Barium chloride solution reacts with sodium sulphate solution
- Calcium metal is reacted with cold water
- (a) (i) Define
- Ionic equation
- Molecular equation.
(b) Complete and balance the following chemical equations.
(i) KCLO3 →heat
![]()
- Pb (NO3)2 →
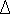 H2SO4 + KOH →
H2SO4 + KOH → - CUCO3 →
- Na + H2O →
- Write balanced ionic chemical equations from the following chemical activities
- Barium chloride solution is mixed with iron (II) sulphate solution
- Dilute hydrochloric acid involves carbon dioxide from sodium hydrogen carbonate
- Magnesium metal dissolves in dilute nitric acid
- Complete and balance the following chemical equations
KClO(3)→Mn O2(g)
- Ca(OH)2(aq) + Ca(HCO3)2(aq) →
- ................... + → CaO(s) + H2(g)
- (a) Complete and balance the following chemical equations
- Ca(s) + O2(g) →
- Mg(s) + HCl(aq)→
- NaOH(aq) + H2SO4(aq) →
- H2O2(l)MnO2 →
- → Ca(NO3)2(aq) + H2O + CO2
- BaCl2(aq) +→BaSO4(s) + HCl (aq)
- CaO(s) + HCl(aq) →
(b) Write balanced ionic equations of the reactions in 6 a (ii), 6 a v and 6 a vi above
- (a) Define the term a chemical equation.
(b) Write down components of a chemical equations list only three (3).
(c) Balancing the following chemical equations;
- Al(NO3)3(aq) + 3NaOH(aq) → Al(OH)3(s) + NaNO3(aq)
- Ba(NO3)2(aq) + (NH4)2SO4(aq) → BaSO4(s) + NH4NO3(aq)
- CaCl2(aq) + Na2CO3(aq) → NaCl(aq) + CaCO3(s)
- Zn(NO3)2(aq) + Na2S(aq) → NaNO3(aq) + ZnS(s)
- Na2CO3(aq) + HNO3(aq) → NaNO3(aq) + H2O(l) + CO2(g)
- (a) Write ionic equation for the following reactions;
- NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
- AgNO3(aq) + NaCl(aq) → AgCl(ls) + NaNO3(aq)
- CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(q) + H2O(l)
- Zn(s) + CUSO4(aq) → ZnSO4(aq) + CU(s)
- a) Differentiate between the following terms.
- Chemical reaction and chemical equation.
- Reactants and products
- Displacement reaction and Double displacement reaction.
b) Complete and balance the following reaction.
- + → Zn(NO3) (aq) + Mg(s)
- Pb(NO3)2 (s) + Na2SO4 (aq) →
- Pb(NO3)2 (s) Heat→
- Study the following equation and answer the questions which follow;
NaOH(aq)+ HCl(aq)→NaCl(aq) + H2O(l) + 57kJ.mol-1
- Name the above equation
- Explain whether or not the reaction is exothermic or endothermic.
- Draw an energy level diagram for the above named reaction.
- Complete and balance the following equations. Show the state symbols;
- Zn + Cl2 →
- CaO + H2O →
- MgCl2 + AgNO3 →
- Study the chemical reaction shown below and answer the questions that follow;
![]() 3KOH(aq) + FeCl3(aq)3KCl(aq) + Fe (OH)3(s)
3KOH(aq) + FeCl3(aq)3KCl(aq) + Fe (OH)3(s)
- Write the total ionic equation for the above chemical equation.
- Write the net ionic equation for the above chemical equation.
- What type of reaction is represent by the equation above?
- Complete and balance the following chemical equations:-
- Fe(OH)3 + H2 SO4 →
- MgCO3 + HCl →
- Na + O2 →
- H3PO3 + Al2O3 →
- Explain the meaning of each of the following types of chemical reactions and support your explanation with the help of a relevant chemical equation.
- Synthesis (combination) reaction
- Decomposition reaction
- Precipitation reaction
- Single displacement reaction
- Neutralization reaction
- (a) Define:-
- Redox reaction
- Oxidation reaction
- Reduction reaction
(b) Classify the following reactions into oxidation and reduction reactions:-
- Write ionic equations from the following chemical reactions:
- Magnesium reacts with dilute sulphuric acid
- Silver nitrate solution reacts with iron (II) chloride solution
- Dilute solution acid reacts with solid calcium carbonate
- Iron displaces copper of copper (II) sulphate solution
- Chlorine gas displaces iodine of potassium iodide solution
- Write the product and balance the following chemical equations
- Write word equations for the following reactions:
- Reaction of zinc granules with Copper (II) sulphate
- Dry hydrogen gas is passed over heated Lead (II) oxide.
- Reaction of Sodium and Chlorine.
- Reaction of Zinc oxide with dilute nitric (VI) acid.
- Dissociation of Calcium carbonate by Heat.
- Reaction of Lead (II) nitrate solution with sodium chloride solution.
- Write formulae equation for the above reactions, then balance.
- Write balance ionic equation of the following chemical reactions:
- Solution of potassium iodine and lead nitrate are mixed together
- Dil sulphuric acid is reacted with magnesium ribbon
- Silver nitrate solution is reacted with aqueous sodium chloride
- Sodium carbonate solution removes calcium ions to solve the problem of permanent hardness of water
- Hydrochloric acid solution neutralizes with sodium hydroxide solution
1.6TOPICAL QUESTIONS ON EQUATIONS
FORM THREE CHEMISTRY.
SECTION A. 20 MARKS.
1. MULTIPLE CHOICE QUESTIONS
 The equation X2+ + 2e-X, represent:-
The equation X2+ + 2e-X, represent:-
- Oxidation reaction
- Reduction reaction
- Displacement reaction
- Synthesis reaction
- Neutralization
- The reaction between Silver nitrate and Sodium chloride to form Silver chloride and Sodium nitrate is an example of a ………………. Reaction.
- Direct combination
- Simple displacement
- Double displacement
- Decomposition.
- Ammonium chloride reacts with sodium hydroxide solution on warming. The net ionic equation for the reaction is.
- H+(aq) + OH-(aq) → H2O(l)
- NH+(aq) + OH-(aq) → NH3(aq) + H2O(aq)
- Na+(aq) + Cl-(aq) → NaCl(aq)
2NH4+(aq) + 2Cl-(aq) → NaCl(aq) + Cl(g) + H2(g)
- The percentage composition of compound K is 53.3% oxygen, 6.7%hydrogen and 40%carbon by mass.The empirical formula is;
- C2H4O
- CH2O
- CH4O
- C2H2O
- If 1g of hydrogen is exploded in air, the mass of water formed is:
- 1.8g
- 9g
- 4g
- 18g
- The ionic equation of the reaction between hydrochloric Acid and Sodium hydroxide is;
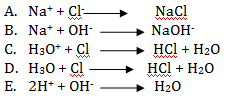
- Write the letter of the best match from column B against a statement in column A.
| LIST A | LIST B |
|
Cu(s) + 2HNO3(aq) → N.R
|
SECTION B.
Answer the questions in this section in brief
- Rewrite complete and balance the following chemical equation of reactions:
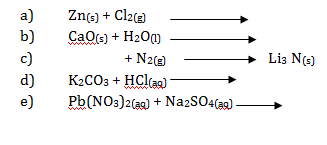
- Write ionic equations from the following chemical reactions:
- Magnesium reacts with dilute sulphuric acid
- Silver nitrate solution reacts with iron (II) chloride solution
- Dilute solution acid reacts with solid calcium carbonate
- Iron displaces copper of copper (II) sulphate solution
- Chlorine gas displaces iodine of potassium iodide solution
- (a) (i) Define
- Ionicequation
- Molecular equation.
(b) Complete and balance the following chemical equations.
(i) KCLO3 →heat
(ii) Pb (N03)2→
(iii) H2 S04 + KOH→
![]() (iv) CUCO3→
(iv) CUCO3→
(v) Na + H2O→
- With the aid of a well balance chemical equations explain what would happen in each of the following reactions
- Potassium carbonate is strong heated
- Concentrated sulphuric acid added to dry salt and heated
- Sodium nitrate heated
- Concentrated sulphuric acid slowly acted to the sugar
- Soluble alkali added to the soluble salts
- Carbon dioxide passed through lime water
- Water is added to a white copper (11) sulphate
- A glowing splint of wood is lowered into a jar of (i) Hydrogen gas (ii) Carbon dioxide gas
- Ammonium chloride is heated
- Dilute nitric acid was heated with (i) warm copper oxide (ii) Zinc carbonate
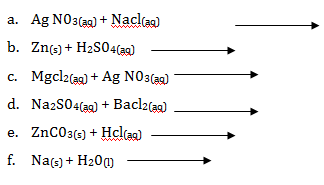
- Write the product and balance the following chemical equations
- Ag N03(aq) + NaCl(aq) →
- Zn(s) + H2S04(aq)→
- MgCl2(aq) + AgN03(aq)→
- Na2S04(aq) + BaCl2(aq) →
- ZnC03(s) + HCl(aq)→
- Na(s) + H20(l) →
- Complete and balance the following chemical equations:-
- Fe(OH)3 + H2 SO4 →
- MgCO3 + HCl→
- Na + O2 →
- H3PO3 + Al2O3 →
- Explain the meaning of each of the following types of chemical reactions and support your explanation with the help of a relevant chemical equation.
- Synthesis (combination) reaction
- Decomposition reaction
- Precipitation reaction
- Single displacement reaction
- Neutralization reaction
- (a) Define:-
- Redox reaction
- Oxidation reaction
- Reduction reaction
(b) Classify the following reactions into oxidation and reduction reactions:-
- S(s) + O2(g) → SO2(g)
- N2(g) + 3H2(g) → 2NH3(g)
- Fe2+ (aq) – e-→Fe3+(aq)
- Fe3(aq) +e-→Fe2+(aq)
(c) Write the balanced equations for the following reactions.
(i)Hydrogen + chlorine→Hydrogen chloride
(ii) Magnesium oxide + carbon→Magnesium + carbon monoxide
(iii)Silver nitrate + sodium chloride→silver chloride + sodium nitrate
- (a) Explain the meaning of “an ionic equation”
(b) Write an ionic equation for each of the following:
- Carbon dioxide gas dissolves in an aqueous solution of potassium hydroxide
- The reaction between magnesium and dilute hydrochloric acid.
SAMPLE NECTA QNS.
2009.
1. (a) Balance the following equations:
(i)Ca + H3PO4→ Ca3(PO4)2 + H2
- Cu + HNO3 → Cu (NO3 )2 + NO2 +H20
- SnCi2+FeC13→SnC14+FeCI2
2016.
QNS.5(a) Give the name of the types of reaction represented by each of the following chemical equations.
- C3H8(g) +50,(0)→ 3CO2 + 4H20(1)
- 2Pb (N 03),(,)→2Pb0(,) + 4NO2 +02(g)
(iii)Zn(s)+CuS04(aq) —>ZnSO4(aq) +CU(S)
2017.
QNS10.(a) Complete the following equations and determine the type of chemical reaction involved in each case.
(i) Zn(s)+ H2SO4(aq)→
(i) AgN 03(aq) + NaCl(aq)→
(iii) N2(g) + H2(g) →
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






