PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM TWO
MID-TERM EXAMS – AUG/SEPT – 2024
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 MARKS)
Answer ALL questions.
i. Which one of the following represent the chemical combination of substances result into the formation of water;
- Magnesium + oxygen → Magnesium oxide.
- Lead (II) Oxide + Hydrogen → Lead + water
- Hydrogen + Oxygen → Water
- Silver oxide + Hydrogen → Silver + Water
ii. What is so special with Francium and Fluorine compared to other elements in the periodic table?
- Francium is a liquid and Fluorine is a gas
- Francium is in group 1 and Fluorine in group 7
- Francium is in periodic 7 and Fluorine in period 2
- Francium is most electropositive and Fluorine most electronegative.
iii. A mixture of two solid substance is commonly heated in the laboratory to produce oxygen such mixture could be that of:-
- Manganese dioxide, hydrogen and magnesium
- Potassium permanganate and magnesium oxide
- Mercury (ii) oxide and hydrogen peroxide
- Potassium chlorate and manganese (iv) oxide
iv. What type of displacement is done by collecting pure hydrogen in the laboratory;
- Downward displacement of water
- Downward displacement of air
- Upward displacement air
- Upward displacement of air
v. What is kindling temperature
- A kind temperature
- Temperature out of a burning material
- The highest temperature obtained from a burning substance
- The lowest temperature at which a combustible material can catch fire.
vi. Which element will form a compound of the formular M2O3 where M is a metal?
- Aluminium and Oxygen
- Beryllium and chlorine
- Oxygen and Sodium
- Calcium and oxygen.
viii. What is so unique about a hydrogen atom on comparing it with other elements?
- It has no neutron in its nucleus
- It has a small relative atomic mass
- It forms a low density gas
- It has no exact place in the Periodic table
ix. Identify the skill not acquired during chemistry study
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
x. When one wants to light the Bunsen burner, what is the first thing to do;
- Light a match/wooden splint and hold it at the gas tap using the rubber tubing.
- Close air hole and connect the burner to the gas tap using the rubber tubing.
- Open the gas tap slowly to half way to fully open position.
- Open the hole slowly
2. Match the items in List A with their corresponding responses in List B.
| LIST A: | LIST B |
|
|
SECTION B
- (a) List down three (3) sources of natural water.
(b) Explain why water is NOT used to extinguish class E fires.
(c ) Give a reason to support the following facts
- Water is universal solvent
- Oxygen is collect over water
- Oxy-hydrogen used in welding
4. The following are atomic and ionic radii (in nm) of members of the same group of the periodic table use the information to answer the questions that follow. The letters do not represent the actual
| Element | Atomic radii (nm) | Ionic (nm) |
| A B C D E | 0.157 0.216 0.133 0.235 0.203 | 0.098 0.149 0.078 0.165 0.133 |
- Is this a group of metallic or non metallic elements? Explain your answer?
- State the element that would have lowest atomic number.
- State the element which would be the most reactive. Give a reason for your answer.
- State the element which would be the most reactive. Give a reason for your answer.
5. Gas “P” has the following properties; it is highly flammable, readily combines with other elements, readily reacts with other chemical substance and is a strong reducing agent.
- Name the gas “P”
- What is the method used to collect gas “P” in the laboratory? Give reason
- Give four (4) uses of gar “P”.
6. (a) write down the chemical formula of the following compounds
- Copper (II) nitrate _____________________________
- Sodium hydrogen carbonate _________________________
- Aluminium chloride _______________________________
(b) Write the IUPAC names of the following chemical compounds:
(i) H2SO4 ________________________________________________
(ii) HCl O3 __________________________________________________
(iii) Cu2O ____________________________________________________
7. (a) Which are the three sub – atomic particles;
- ……………………………. (ii) …………………………….. (iii) ……………………............
(b) Which sub – atomic particles from the nucleus and what is their common name?
Particles (i) ……………………………. (ii) …………………………………………
Common name …………………………………………………….
8. (a) Define:- (i) Valency (ii) oxidation state (iii) Radical.
(b) Calculate the oxidation number of the underlined elements:-
(i) Na2SO4 (ii) SO42- (iii) K2Cr2O7 (iv) NH4+ (v) MnO4-
9. (a) Define the following terms;
- Water treatment ………………………………………………………………………………………………………………………………………………………………………………
- Water purification …………………………………………………………………………………………………………………………………………………………………………………
(b) Name impurities that can be found in water?
- …………………………………………………………………………
- …………………………………………………………………………
- …………………………………………………………………………
- …………………………………………………………………………
(c) State any two methods of domestic water treatment;
(i) ……………………………………………………………………………..
(ii) …………………………………………………………………………….
SECTION C
10. (a) What is the suitable term in chemistry used for the tendency of an atom to attract an
electron pair towards itself? …………………………………………………………
(b) Can isolated atoms show the same behaviour? …………………………………….
(c) Which is the suitable term in chemistry used in opposite to the 7(a) and (b) behaviours above? …………………………………………………………………
………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 183
FORM TWO CHEMISTRY EXAM SERIES 183
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM TWO
MID-TERM EXAMS – AUGUST – 2023
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 MARKS)
- For each of items (i) – (x) choose the correct answer among alternatives given.
- Factors in an experiment that can be manipulated to get desired results is called
- Controlled variable
- Manipulated variable
- Dependent variable
- Independent variable
- What might happen to a careless pupil in the laboratory
- He may get injures
- He will not achieve in studies
- He will cause loss of things
- Nothing will happen
- The formation of water when hydrogen bums in Oxygen shows that
- Hydrogen is an element
- Air supports combustion
- Water is Oxide of hydrogen
- Water is an product of combustion
- Which criterion has been used to assign the symbol Ag of the element?
- The use of an English name to the element
- The use of a Latin name is the element
- Capitalizing first letter, then third letter small from the Latin name.
- Capitalizing first latter then third small from an English name.
- The moving solvent in the chromatographic column is called _________
- Mobile phase
- Stationary phase
- Analyte
- Chromatogram
- What does it indicate when the match head on base of Bunsen Burner flame does not ignite or burn
- The gas is not enough at that point
- The gas is not burnt at that point
- The match head easily bums when subjected to friction
- The match head has not be pushed on the flame from outside
- Why is covering iron or steel interferes with the rusting of iron?
- It cuts off oxygen
- It prevents direct contact with water
- It prevents contact of iron or steel with oxygen and with water
- It extends the time required by iron to rust
- The property of Oxygen that can be used for its test is
- It is colorless and odorless
- It is lighter than air
- It relights a glowing splint
- It is slightly soluble in water
- A certain liquid dissolves copper (II) Sulphate to form a blue solution, this liquid is likely to be
- Hydrochloric acid
- Liquid Oxygen
- Nitric acid
- Water
- Isotopes of Bonon appear as 10B of 20% abundance. What would be its average relative atomic mass?
- 10.80
- 10.08
- 10.88
- 10.82
- Match the description in LIST A with the correct response from LIST B by writing the letter of the correct response in the space provided.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section
- (a)A stone is said to be a good example of matter Give two reasons to support this fact.
(b)With reasons explain why it is necessary to have the following in Laboratory
- Laboratory door open outward
- Laboratory floor is rough and never polished
- Bucket of sand is found near petrol station
- (a)A student aimed to prepare gas G by using moderate reactive metal with a dilute acid. By using the information given above, answer the following questions.
- What is the name of gas G?
- What is the chemical test that distinguishes this gas from other gases
- With two reasons, state the correct means of collecting the gas?
- Write the balanced chemical equation for the reaction
(b)Juma wanted the above reaction to go fast because wants to utilize in venous industrial uses. You as a chemist provide him four means that can be used to make reaction faster.
- (a)T and K are elements found in the periodic table. The atomic number of T is 16 and that of K is 19
- In which group and period of periodic Table does element T and K appear?
- Write the chemical formula of a compound formed between T and K
(b)(i) Which particles are atoms of the same element in the list of the particles given below?
![]()
(ii) Why can’t neon react with sodium?
- (a)Define molecular and Empirical formula?
(b)A compound contains 20% by mass mg, 26% of sulphur and Y% of Oxygen
- Find empirical formula of the compound
- Find its molecular formula.
- Study the periodic table below
| I VIII | |||||||
| A | II III IV V VI VII | B | |||||
| C |
| D |
|
|
|
| E |
| F |
|
|
|
| G | H |
|
|
| J |
|
|
|
|
|
|
Use the letters shown in the periodic table above to indicate
- Element with Zero valence
- The lightest atom
- The alkali, earth metal
- An element with the electronic configuration of 2:8:1
- Give the names of element represented by the mentioned letters, A, B, C and D
- give the name of J on an element
- the gaps left out by _____ periodic table is now filled by discovered _______, ______, _____ and the artificially made elements
(b)Write valence of following radicals
- Ammonium
- Magnesium sulphate
- (a)List down three (3) sources of water
(b) Explain why water is not used extinguish class E fires
(c)Give a reason to support the following facts
- Water is universal solvent
- Oxygen is collected over water
- Oxy-hydrogen is used in welding
- (a)Mariam was preparing food for her family using hot oil in frying pam. Accidentally the Pam tripped over and huge fire spread over her kitchen floor.
- Mention two fire extinguishers which would be appropriate to like when trying to put out the fire?
- Which fire extinguisher would be dangerous to use.
(b)Mention three conditions for fire to start
- (a)Briefly explain any five methods of preventing rusting
(b)A certain pink colored compound is heated to form blue colour. When water is added to sample, it changed back to pink
- What change is demonstrated by the compound
- Explain reason for your answer.
FORM TWO CHEMISTRY EXAM SERIES 154
FORM TWO CHEMISTRY EXAM SERIES 154
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM TWO EXAMINATION – SEPTEMBER 2022
032 CHEMISTRY
TIME: 2:30 HOURS
INSTRUCTIONS
- This paper consists of sections A and B with a total of TEN (10) questions
- Answer all questions in the space provided.
- All writing must be in black or blue ink except for diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Zn= 65, X =32, O=16
SECTION A (15 Marks)
Answer all questions in this section
- For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the bracket provided.
- When burning a fuel produces blue color it means there is
- Adequate supply of oxygen with production of soot
- In adequate supply of oxygen with production of soot
- Adequate supply of oxygen with production of less heat
- Adequate supply of oxygen with production of more heat ( )
- Which of the following is an agricultural chemical products made by the application of chemistry?
- Drugs B. Pesticides C. Clothes D. Cement ( )
- Which of the following is NOT among the composition of air?
- Noble gases B. Hydrogen C. Carbon dioxide D. Nitrogen ( )
- Which one of the following sets of laboratory apparatus are used for measuring volume of liquids
- Conical flask, measuring cylinder and test tube
- Burette, pipette and measuring cylinder ( )
- Volumetric flask, water though and burette
- Gas jar, measuring cylinder and pipette
- The following are the characteristic of ionic compound
- They are easily vaporized
- They are easily dissolved in organic solvent
- Conduct electricity when in solution or molten
- Usually exist as liquid or gas at room temperature
- Which of the following is a symbol of silver ion
- Ag B. S C. Ag+ D. Si ( )
- What is proton, electron and atomic number AI ( )
- 13,13,13 B. 13,11,13 C. 13,12,13 D. 13,13,11
- Which state is involved when drying wet clothes?
- Liquid to solid B. Gas to liquid C. Liquid to gas D. Solid to gas( )
- The components of fire triangle are ;
- Oxygen, fuel and heat C. Oxygen, heat and hydrogen ( )
- Oxygen, nitrogen and heat D. Oxygen, carbon dioxide and fuel
- Which group and period does the element with 14 electrons belong?
- Group II and period 3 C. Group III and period 3 ( )
- Group IV and period 3 D. Group II and period 4
- Match the descriptions in List A with the corresponding in List B by writing the letter of the correct response beside the item number in the answer booklet provided
| LIST A | LIST B |
|
|
Answers
| LIST A | i | ii | iii | iv | v |
| LIST B |
|
|
|
|
|
SECTION B (70 Marks)
Answer all questions in this section
3(a) Fill the blanks
- The techniques used to separate serum from blood samples is called _________________________
- The insoluble substances remain in a filter paper during filtration are termed as ___________________________
- Boiling points of substance reflect the strength of __________________
- The sub atomic particles of an atom are ____________________ and _____________________
- Solar energy is example of _______________________resources
- (b) Suggest the best method of separating the following mixture
- Alcohol and water _______________________________________________________________________________________________________________________________________________________________________________________
- Sodium chloride and water _______________________________________________________________________________________________________________________________________________________________________________________
- Green solution from leaves _______________________________________________________________________________________________________________________________________________________________________________________
- Sand from rice _______________________________________________________________________________________________________________________________________________________________________________________
- Iron fillings and powder calcium carbonate _______________________________________________________________________________________________________________________________________________________________________________________
- (a) Complete the following table
| Class of fire | Materials | Fire extinguisher | Chemical composition of extinguisher |
| CLASS A |
|
| Ordinary tap water pressurized by air |
|
| Flammable liquids | Dry powder extinguisher |
|
| CLASS C |
| Sand bucket
|
|
|
| Metal |
| Sulphuric acid and sodium hydrogen carbonate |
| CLASS E |
|
| Protein and fluoro protein |
(b) With one example explain each of the following
- Alkali earth metals __________________________________________________________________________________________________________________________
- Metalloids __________________________________________________________________________________________________________________________
- Transition metals __________________________________________________________________________________________________________________________
- Why are noble gases stable? __________________________________________________________________________________________________________________________
- Why covalent compound do not conduct electricity __________________________________________________________________________________________________________________________
- (a) What is destructive distillation
__________________________________________________________________________________________________________________________________________
(b) Mention five (5) procedures of preparing charcoal from the method mentioned in
5(a) above
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
(c) Mention four (4) steps of lightning a Bunsen burner
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- An atom of element X having atomic number 11 combines with an atom of element Z having atomic number 17 to form a compound
- Write the formula of the compound and state the type of bond formed in the compound
- Give four properties of compound in 6(a)
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
- (a) Write the chemical symbols for beryllium, boron, neon, nitrogen and phosphorus
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
(b) You are provided with a compound composed of 40.5% zinc. 19.6% sulphur and
39.9% oxygen. Calculate the molecular and empirical formula. If it’s molecular
mass is 161
- (a) By giving one reason, explain the following facts
- During laboratory preparation of oxygen gas, little manganese dioxide is added to hydrogen peroxide.
__________________________________________________________________________________________________________________________
- Fish can obtain oxygen for respiration although spend their life in water
_________________________________________________________________________________________________________________________
- Oxygen gas can be used for welding activities although it does not burn
__________________________________________________________________________________________________________________________
(b) Which property enables the use of hydrogen gas in
- Fueling __________________________________________________
- Manufacturing of margarine _________________________________
- Give two domestic uses of oxygen gas
- ________________________________
- ________________________________
- (a) Define the following terms
- Water treatment __________________________________________________ ________________________________________________________________
- Water purification _____________________________________________ ________________________________________________________________
(b) List down four (4) economic uses of water
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
(c) What are the importance of water treatment and purification? Give two (2) reasons
__________________________________________________________________________________________________________________________________________
SECTION C (15 Marks)
- Briefly explain five methods used to prevent rusting and give example for each
Page 1 of 6
FORM TWO CHEMISTRY EXAM SERIES 111
FORM TWO CHEMISTRY EXAM SERIES 111
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM TWO- AUG/SEPT 2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
(i) The elements which are found in group VIII of the periodic table are known as:
- Metals
- Noble gases
- Non - metals
- Right elements
(ii) Isotopes are atoms which have:
- Different mass number
- Different number of electrons
- Different number of protons
- The same number of neutrons
(iii) The elements which are found in group VIII of the periodic table are known as:
- Metals
- Noble gases
- Non - metals
- Right elements
(iv) The process used to separate a mixture of salt and water is:
- Evaporation
- Filtration
- Simple distillation
- Sublimation
(v) Saturated solution is one which:
- Contains more solute undissolved at a given temperature.
- Will take no more of solute at a given temperature.
- Contains a little solute at a given temperature.
- Has a large amount of solvent at given temperature.
(vi) Which of the following electronic configurations are of metals?
- 2:8:8:1 and 2:8:8:7
- 2:8:3 and 2:8
- 2:8:8:1 and 2:8:3
- 2:8:6 and 2:8:8:7
(vii) One isotope of an element has atomic number A and mass number M. How many neutrons are contained in the nucleus of its atom?
- M
- A
- A - M
- M - A
(viii) The total number of protons and neutrons in the nucleus of an atom is called:
- Valency number
- Atomic number
- Molecule number
- Mass number
(ix) Oxidation may be defined as:
- Loss of hydrogen by a substance
- Gain of hydrogen by a substance
- Reaction in which oxygen is lost
- Reaction in which electrons are increased
(x) Moving across a period in the periodic table,
- Electro negativity decreases
- Electro negativity increases
- Metallic property increases
- Electro positivity increases
2. (a)Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| LIST A | LIST B |
|
|
(b) Answer the given questions by writing the correct answer in the blank spaces provided.
- Has both metallic and non metallic properties………………………
- A gas that can undergo sublimation………………………..
- Name given to group seven elements which means salt producer…………………..
- An example of renewable fuel from organic matter…………………..
- Separation of colored substances…………………..
3. Briefly explain five characteristics to be considered when looking for a good fuel.
4.(a) During preparation of Hydrogen gas by the reaction between dilute Hydrochloric acid and Zinc granules, the granules slowly dissolve in acid to form solution X.
(i) Name solution X .........
(ii) Write the chemical formula of X . . . . . .
(b) How can hydrogen gas be tested?![]()
(c) Mention four (4) chemical properties of hydrogen gas.
(d) List three (3) uses of Hydrogen gas.
5.Outline six common apparatus used in the laboratory preparation of oxygen gas using hydrogen peroxide.
(b) Outline four uses of oxygen in everyday life situation.
6.(a) Define the following terms:
(i)Valency
(ii)Oxidation state
(iii) Anion
(iv)Cation
(b) Calculate the oxidation state of the underlined elements in the following radicals:
(i) NH4+
(ii) SO42+
(iii) CLO3-
(c) A compound consists of 40% carbon, 6.67% hydrogen and 53.33% oxygen. If its relative molecular mass is 60, calculate the following:
(i)Empirical formula
(ii)Molecular formula
7.(a) Define the following terms:
(i)Oxidation state ![]()
(ii)An element .......„
(iii)A compound
(iv)Fainting ![]()
(b)Write the chemical formula for each of the following compounds:
(i)Sodium sulphate ![]()
(ii)Sodium chloride .. ![]()
(iii)Calcium nitrate ......... (iv) Calcium oxide . ![]()
8.State two chemical properties of water.
(b) Calculate the molar mass of each of the following compounds:
(ii) NaHC03 (iii) Fe203 ![]()
9.Define the following terms:
(i)Empirical formula .........
(ii)Molecular formula .........
(b)A compound consists of 85.7% carbon and 14.3% hydrogen by mass. If its relative molecular mass is 56, calculate:
(i)Empirical formula.
(ii)Molecular formula.
10.(a) Study the following periodic table and then answer the questions that follow:

(i) Name elements named by the following letters: S, W, X, Z
(ii) Write the electronic configuration for the elements represented by the following letter.
10.(b) Study the experiment diagram below and answer the questions that follow.
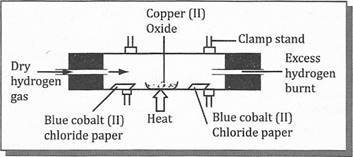
(i)What happens to the copper (Il) oxide during the experiment?
(ii)What happens to the two pieces of cobalt paper? (iii) Write a word equation for the reaction.
FORM TWO CHEMISTRY EXAM SERIES 65
FORM TWO CHEMISTRY EXAM SERIES 65
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
AUGUST-SEPTEMBER EXAMINATION SERIES
CHEMISTRY FORM-2
2020
TIME: 2:30 HRS
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) Chemistry is defined as:
- The scientific study of matter, compounds and chemical reactions
- The scientific study of compounds, mixtures and organic substances
- The scientific study of composition, structure and properties of matter
- The study of relation between human being, medicine and pollution.
(ii) A non-luminous flame is the most applicable flame for heating purposes because:
- It is very noisy
- It has no soot
- It is very hot
- It has no colour
(iii) Matter is defined as anything that has:
- Volume and occupies space
- Mass and occupies space
- Mass and occupies density
- Density and space
(iv) Which of the following process is used in preventing rust of an iron?
- Water
- Boiling
- Salting
- Galvanization
(v) Water is a universal solvent because:
- It is available everywhere
- It boils at 1000C
- It dissolves most of the solutes
- It dissolves all crystals in compounds
(vi) Which among the following are the two processes involved during distillation?
- Evaporation and sublimation
- Evaporation and crystallization
- Evaporation and condensation
- Evaporation and decantation
(vii) Which of the following set of nuclide notation represents isotopes?
- 18 X 16 X 19 x
- 18 X 18 X X
- 16 x 18 x 1890x
- 16 X 1789x,
(viii) The chemical used to test the presence of water in a substance is:
A. Cobalt Il oxide B. Cobalt 111 oxide
C. Cobalt chloride D. Copper Il chloride
![]() (ix) When a burning fuel produces blue colour it means there is:
(ix) When a burning fuel produces blue colour it means there is:
A. adequate supply of oxygen with production of soot
B. inadequate supply of oxygen with production of more heat.
![]() C. inadequate supply of oxygen with production of soot.
C. inadequate supply of oxygen with production of soot.
D. adequate supply of oxygen with production of more heat.
![]() (x) Which of the following is the best apparatus for measuring accurately a fixed volume of a given solution?
(x) Which of the following is the best apparatus for measuring accurately a fixed volume of a given solution?
A. Measuring cylinder B. Beaker
C. Pipette D. Burette
2. Match each item in List A with a correct response in List B by writing its letter bellow the number of the corresponding item in the table provided.
| LIST A | LIST B |
| A. Metalloids B. Non-metals C. Periodicity D. T r a n s i t i o n elements E. Electro negativity F. Alkali metals G. Halogens H. Periodic law I. Alkali earth metals J. Rare non metals K. Period L. Noble gases M. Periodic table N. Group |
SECTION B. 80 MARKS
3.(a) Define the following terms:
(i)Oxidation state ![]()
(ii)An element .......„
(iii)A compound
(iv)Fainting
(b)Write the chemical formula for each of the following compounds:
(i)Sodium sulphate ![]()
(ii)Sodium chloride .. ![]()
(iii)Calcium nitrate ......... (iv) Calcium oxide .
4.Gas X can be prepared in the laboratory by the decomposition of hydrogen peroxide.
(a)Identify gas X .. ![]()
(b)State three physical properties of Gas X
![]() Mention three chemical properties of gas X. (d) State three uses of gas X.
Mention three chemical properties of gas X. (d) State three uses of gas X.
5.Write the name of each of the following compounds:
(i)(NH4)2C03
(ii)CaCl2
(iii) NaSO4
(iv) KC103
(b)Give three differences between the following:
(i) ![]() Electrovalent compounds and covalent compounds. (ii) Solutions and suspensions.
Electrovalent compounds and covalent compounds. (ii) Solutions and suspensions.
6.(a) Define the following terms:
(i)Covalent bond
(ii)Electrovalent bond
(b) A compound consists of 82.8% carbon and 17.2% hydrogen by mass. The vapour density of the compound is 29. Calculate:
(i)Empirical formula.
(ii)Molecular formula.
7.(a) Define the following terms as applied in Chemistry:
(i)Flame ![]()
(ii)Bunsen burner
(iii)Laboratory
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
8.(a) Write a word equation for each of the following reactions:
(i)Calcium burns in Oxygen .........
(ii)Sodium reacts with water ... ... ...
(b) What do you understand by the following terms?
(i) Water treatment
(ii) Water purification
(c) Mention six uses of water in economic activities.
9.(a) Mention four physical properties of water.
(b) What will happen when:
(i) a burning splint of wood is introduced into a gas jar containing oxygen gas
(ii) oxygen gas reacts with metals .
(iii) hydrogen gas reacts with oxygen gas .........
(c) List four uses of hydrogen in our daily life.
10. What do you understand by the term "valency"?
(b) Calculate the oxidation number of the underlined elements: (i) NaOH
(i) CO3
(iii) Na3P04
(iv) NO2
(v) S02
(c) State the importance of changes of state in daily life.
FORM TWO CHEMISTRY EXAM SERIES 31
FORM TWO CHEMISTRY EXAM SERIES 31
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







