THE OFFICE OF THE PRESIDENT, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT.
SECONDARY EXAMINATION SERIES
MARCH 2025
CHEMISTRY FORM THREE
TIME: 2:30HRS
INSTRUCTIONS
- This paper consists of three section A, B and C with a total of eleven (11) questions.
- Answer all questions from section A and B and two questions from section C.
- Section A carries sixteen (16) marks, section B carries fifty four (54) marks and section C carries thirty (30) marks.
- All writing should be in blue ink except diagrams which must be in pencil.
- All communication devices and any unauthorized material are not allowed in the examination room.
- Write your Examination number at the top right corner of every page.
SECTION A (16 Marks)
(Answer all questions in this section)
1. For each of the items (i –x) choose the most from the given alternatives and write its letter beside the item number in the answer sheet (booklet) provided.
i. Which of the following is not a use of a solvent?
- Bleaching agent
- Greasing.
- Stain removal.
- Cleaning
- Universal solvent
ii. In which step of scientific procedures does the hypothesis is either proved or disproved?
- Data collection and analysis.
- Experimentation.
- Data interpretation
- Formulation of hypothesis.
- Conclusion
iii. Which of the following groups consist of home Care products?
- Yeast, plastic and disinfectant.
- Clothes, soap and stone.
- Air freshener, detergent and antiseptic.
- Petrol, air freshener and paints.
- Air freshener, detergent and disinfectant
iv. An electric current of 0.2A was passed through an electrolyte for 16.67minutes. The quantity of electricity passed is;
- 200.04cuolombs
- 2000.004cuolombs
- 1000cuolombs
- 0.254cuolombs
- 0.00789culombs
v. What is the correct meaning of ionization energy?
- Energy required to remove an electron from the inner most shell
- Energy required to remove electron from the outer most shell
- Energy required to add electron to the inner most shell
- Energy required to add electron to the outer most shell
- Energy required to attract electron towards the nucleus of an atom
vi. An increase in temperature of a gas in enclosed system/container causes an increase in pressure of the gas. This is because it increase the.
- Number of gas molecules
- Combination of gas molecules
- Number of collision between gas molecules
- Average velocity of a gas molecules
- Kinetic energy of the gas particles
vii. Which of the following is not the use of chromatography?
- To analyze blood in crime scenes
- To detect different fibres
- To detect water pollution
- To bleach dye or colour
- To test purify of organic substance
viii. Which of the following pairs constitute the best methods for treating and purifying water?
- Chlorination and aeration
- Chlorination and decantation
- Chlorination and filtration
- Chlorination and sedimentation
- Chlorination and distillation
ix. 1.4g of potassium hydroxide is dissolved in water to form 250cm3 of Solution.
What is the Molarity of this solution?
- 0.001M
- 0.1M
- 1.4M
- 5.6M
- 6.0M
x. An electric current was passed through a concentrated solution of hydrochloric acid using carbon electrodes. The substance liberated at anode was.
- Copper
- Hydrogen
- Oxygen
- Sodium
- Chlorine
2. Match the items in LIST A with the responses in LIST B by writing the letter of the correct response besides the item number in the answer sheet provided
| LIST A | LIST B |
| (i) Biogas (ii) Biomass (iii) Natural gas producer gas (iv) Water gas (v) Ethanol |
|
3. Aisha drew a periodic table and then put a shadow on the element with atomic number 8
(a) What type of chemical bond is found between atoms of the element?
(b) Compound X contains 24.24% Carbon, 4.04% Hydrogen and 71.72% Chlorine.
Given that, the vapour density of X is 49.5.
(i) Calculate molecular formula of the compound X
4. (a) Write ionic equation for the precipitation of Barium sulphate from Barium chloride and Sodium sulphate
(b) It is not advisable to sleep inside the house which is not well ventilated with a burning wooden charcoal. Give a reason for that and write the chemical equation to support your answer.
(c) Consider the following elements of group seven n order of which they appear in their group in the periodic table F, C 1, Br, and I
- Which element is more electronegative
- Name the least electronegative element
- Which element has the largest atom?
- Write the electronic configuration of the chlorine atom (9marks)
5. (a) Distinguish between Temporary Hardness and Permanent Hardness of Water basing on their ions.
(b) By use of equations, show how each of the type of hardness in (a) above can be eliminated.
(c). Giving four reasons, explain why people who use hard water can expect high costs than people who use soft water. (9 marks)
6. (a)Catherine is planning to make fire for cooking ugali for her family. What are necessary conditions which must be present so that she can make fire successfully for cooking ugali for her family?
(b)Give for each of the followings
- Water is universal solvent
- Some metal like zinc do not get rust.
- Chlorine gas is collected by downward delivery
- Carbon dioxide turns lime water into milky colour.
7. (a)What do you understand the term chemical equation?
(b)Write ionic equation for the following chemical reactions.
- Milk of magnesia is used to relieve indigestion
- A divalent metal displacing monovalent metal from its soluble nitrate
8. (a) Differentiate the temporary hardness of water from permanent hardness of water basing on their content.
(b) Consider the sample of water taken indifferent places at Arusha region. The samples were boiled and treated with the soap while others were treated with soap before boiling to obtain different results:
| Sample Of Water | Unboiled | Boiled |
|
| Volume of Soap used(cm3) | Volume of Soap used(cm3) |
(i) Which sample contains temporary hardness of water? (Give reason)
(ii) Which sample contains permanent hardness of water? (Give reason)
(iii) What do you think will be the cause of variation of volume of soap of Maroroni water?
(c) Mention the methods (s) used to soften sample of water at Njiro.
9. Element R having atomic number 20 combines with element S having atomic number 17 to form a certain compound
(a)Write the formula of the compound and state the type of bond formed in the compound
(b) Give any three properties of the compound formed in 7(a) above
10. a) How many chlorine molecules are in 20cm3 of chlorine gas at s.t.p.
b) Calculate number of ions present in 5g of copper (II) Nitrate
10. (a) Gas A was prepared in the laboratory by isolating it from atmospheric air. During it's preparation air was allowed to pass through sodium hydroxide then over heated copper metal.
(i) Identify gas A.
(ii) By using equation, explain what happened when gas A passed through sodium hydroxide and in heated copper metal.
(iii) Write two uses of gas A
(b) (i) What will happen when copper is strongly heated in air at higher temperature and lower temperature respectively.
(ii) Explain how the pure copper is obtained from its ore?
SECTION C. 15 MARKS
11. (a) Makame’s daughter was sick. When he took her to the hospital, she was
Prescribed some medicine including a bottle of syrup. The bottle was written “shake well before use”.
(i) What does this statement signify?
(ii) Give two differences between the prescribed medicine in 10(a) above and the mixture of sugar and water.
(b) Students of mining course at a certain college conducted research on extraction of iron. They found that iron can be obtained from its ore in four stages. Briefly explain to them the four stages they should follow to extract it.
FORM THREE CHEMISTRY EXAM SERIES 193
FORM THREE CHEMISTRY EXAM SERIES 193
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
MID TERM ONE – MARCH-2024
CHEMISTRY FORM THREE
Time: 3Hours
Instructions
- This paper consists of sections A, B, and C with a total eleven (11) questions.
- Answer all question in the sections A, B and two (2) questions from section C.
- Section A carries sixteen (16) marks, section B fifty four (54) marks and section C carries thirty (30) marks.
- All writing should be in blue or black pen, except for diagrams that must be drawn in pencil.
- Communication devices and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet (s
SECTION A: (16 MARKS)
Answer all questions in this section.
- For each of the following items (i-x) choose the correct answer from the given alternatives and write letter besides item number in the answer booklet provided.
- Which of the following is not a use of solvent
- Bleaching agent
- Greasing
- Stain removal
- Cleaning
- Universal solvent
- In which step is scientific procedure does the hypothesis either proved or disapproved?
- Data collection and analysis
- Experimentation
- Data interpretation
- Formulation of hypothesis
- conclusion
- During the steam reforming method in industrial preparation of hydrogen. The steam reacts with what compound to produce Hydrogen gas
- water
- carbon monoxide
- methane
- sulphur dioxide
- oxide
- what will be simplest taste of hardness of water
- shaking water with chalk
- mixing water with soap detergent
- formation of scum
- shaking water with soap solution
- formation of dolomite`
- Which of the following set of process uses a gas that ignites with a pop –sound when a lighted splint is passed through?
- Balloon filling, welding, diving
- Hardening of oil, balloon filling , welding
- Hardening of oil, balloon filling and divings
- Fuelling rocket, diving and welding
- Balloon filling, fuel rocket and diving
- The cause of permanent hardness of water is
- CaCOh)2
- CaCHCO3)2
- Mg (HCO3)2
- Na2SO4
- CaSO4
- Domestic Utensils made of iron must rust easily on a result of presence of
- Air and fire
- Air and water
- Water and oil
- Water and oil
- Water and oil
- Which of the following pairs constitutes the method of treating and purifying water
- Chlorination and Aeration
- Chlorination and Decantation
- Chlorination and Filtration
- Chlorination and Distillation
- Chlorination and sedimentation
- The process of giving away water of Crystallization to the atmosphere by a chemical substance is called
- Efflorescence
- Deliquesce
- Hydroscope
- sublimation
- evaporation
- when water is added to an acid, that acid becomes
- more acidic and its PH goes down
- more acidic and its PH goes up
- less acidic and its PH went down
- less acidic and its PH went up
- neutral and its PH become 7
- Match the properties of element in list A with the respective element in list B by writing the letter of correct response besides the item number in answer sheet provided
| LIST A | LIST B |
|
|
SECTION B
54 MARKS
Answer all questions.
- a) The modern periodic law is based on modification of the Mendeleev Periodic Law. Explain how the two theories differ from each other
b) Comment on the following statement
- lithium has large size than beryllium
- sodium is smaller than potassium
- Give any four ions whose electronic configuration resemble to that of Neon.
- A) Magnesium burns in a carbon dioxide giving white solid and black solid sparks. The white solid dissolves in nitric acid leaving colorless solution R
- Write balanced equation for reaction that give white solid
- Identify solution R
B) Differentiate between suspension and solution
- a) The table below shows two brands of bottled water for drinking and concentration of different mineral ions in each brand. Study the table and answer question that follows
| Composition mg/ litre | mineral | Na+ | Ca2+ | Mg2+ | Cl- | No3-1 | So42- | Fe2+ | F-1 |
|
| Uhai | 40 | 3.05 | 4.15 | 14.18 | 0.48 | 10.0 | 0 | 1.76 |
|
| Dasani | 22.32 | 2.69 | 0.11 | 6.5 | 1.0 | 8.0 | 0 | 0.45 |
- Which brand of water is harder? Explain
- State the benefit of having calcium ions in water
- Tap water is always treated before being used, state what is added to perform that function
b) Hydrogen and phosphorus are non metallic elements
- Which one between the two is more electropositive
- Show your wake clearly write chemical formula and name of compound fotmed when two atoms combined
- A)State the main raw materials and process involved in manufacture of the following products
- Wood charcoal
- Coke
- Lamp black
- Animal charcoal
b) Define the terms below
- Mole
- Molar mass
- A) Students are advised to use non-luminous flames for heating in laboratory.
- Explain how a Bunsen burner produces non- luminous flames
- Give a reason as to why the above flame is best
- Give function of air holes in a barrel of Bunsen burner.
b) Explain why hydrogen is proffered to potassium chloride during preparation of oxygen gas.
- A) different salt behave differently when heated use a balanced chemical equation to show how Carbonate and Sulphate behave when subjected to heat
b) Write a balanced and ionic equation for reaction between sodium carbonate and hydrochloric acid
c) Differentiate ionic equation from molecular equation
SECTION C
30MARKS
- Aisha drew a periodic table and then put a shadow on element with atomic number 8
- What type of chemical bond is found between atoms of the element
- Compound X contains 24.24%, 4.04% hydrogen and 71.72%chlorine. Given that the vapor density of X is 49.5. calculate molecular formula of compound X
- If 0.5 g of hydrogen gas is exposed in air, what mass of water will be formed
- A) give two ways you can use to tell water os polluted
b) We have coal in Kuwira Mbeya region; the government authorities have allowed the use of coal for domestic and industrial purposes. What warning can you raise concerning the likely effects? Give five points
- Consider the diagram below
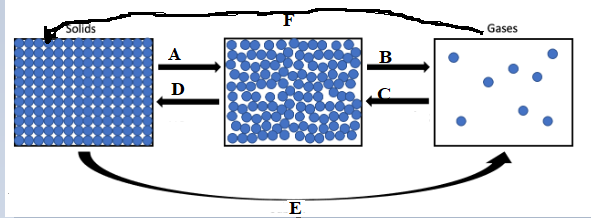
- Give the aim of the above process
- Identify the process of A to F
- Give two importance of above diagram in our daily life
b) State weather the following is permanent change or temporary change
- Dissolution of salt in water
- Rotting of mangoes
FORM THREE CHEMISTRY EXAM SERIES 160
FORM THREE CHEMISTRY EXAM SERIES 160
PRESIDENT OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSESSMENT
032/1 CHEMISTRY FORM THREE
NEW NECTA FORMAT-2023
MID-TERM EXAMS MARCH – 2023
Time: 3:00 Hours
Instructions
- This paper consists of section A, B and C with a total of thirteen (13) questions.
- Answer all questions in this paper
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
- Atomic masses: H=1, C=12, O=16, N=14, Pb=108
- Avogadro’s number = 6.02 x 1023
- GMV at s.t.p = 22.4 dm3
- 1 Faraday = 96,500 coulombs
- Standard pressure = 760 mm Hg
- Standard temperature = 273 K
- 1 litre = 1dm3 = 1000cm3
SECTION A (16 Marks)
1. For each of items (i) – (x), Choose the correct answer from among the given alternative beside item number in answer booklet provided
- The technician prefer to use blue flame in welding because
- It is bright and non-sooty
- It is light and Non-sooty
- It is very hot and large
- It is very hot and non-sooty
- One of the following is not correct about coke being a better fuel than coal as it is
- Does not produce carbon dioxide
- Does not produce poisonous gas
- Has higher heat content
- It is clean and smokeless
- An important property of Oxygen that differentiate it from other gases is
- Bum and support combustion
- Burn but does not Support combustion
- Neither bums nor Support Combustion
- Supports combustion but does not bum
- Hard water which can be softened by boiling method contains dissolved
- Calcium carbonate
- Calcium sulphate
- Magnesium Chloride
- Magnesium hydrogen carbonate
- The source of energy which when used can be made to be put into use again is known as
- Fuel
- Non-renewable energy
- Renewable energy
- Solar energy
- One Isotope of an element has atomic number A and a mass number M. how many neutrons are contained in nucleus of atom?
- M
- A
- A-M
- M-A
- The arrangement of electrons in the atomic number 15 may be represented as follows
- 2:5:8
- 2:8:5
- 2:5:2
- 8:2:5
- Which of these methods can remove both temporary and permanent Gardness of water?
- Using ion exchange chamber
- Boiling
- Using Calcium hydroxide
- Evaporation
- A weak acid is best described on
- An acid that does not ionize completely
- A dilute Acid
- An acid that is harmless
- An acid
- Which is not an advantage of biogas
- It is cheaper source of Energy
- Pollutes the environment
- It is renewable source of energy
- Creates employment to the youth
2. (a)Match the terms in List A with explanation on List B and write the answer on spaces provided.
| LIST A | LIST B |
|
|
SECTION B (54 Marks)
Answer all questions in this section
3.
- How can the society minimize the section encountered in use of charcoal and fire wood?
- Briefly explain the concept of scientific procedure
- What is the importance of scientific procedure in daily life?
4. (a)Give the meaning of the following
- Basicity of an acid
- The pH scale
(b)Categorize the following salts
- PbSO4
- MgHSO4
- Zn(OH)Cl
- MgHCO3
- NH4HSO4
- Ba(NO3)2
5.
- State Avogadro’s hypothesis
- 0.6g of gaseous fluoride is found is occupy 112cm3 at STP. Calculate the relative molecular mass of the fluoride
- 3.5g of hydrated salt, MSO4 x H2O was heated to a constant mass of 3.21g of hydrous salt of anhydrous salt. Calculate the value of (M=63.5, S=32, O= 16, H=1)
6. Soap solution of different amount of water are tested from four different sources and produces lather observed for 30 seconds. The three groups of water were untreated, Boiled and Treated by ion exchange. The results were as follows.
| Sample | Untreated | Boiled | Passed though |
| | 12 | 1.8 | 1.8 |
| | 17 | 17 | 1.7 |
| | 26 | 20 | 1.8 |
| | 1.6 | 1.6 | 1.6 |
Use above results to answer question that follow
- Which is hardest water sample? Why
- Which sample is like distilled water. Explain
- Which chemical substance might cause hardness is (i)Simple A sample B?
- Write an equation for reaction of removing hardness in sample C
7. (a) Define chemical equation and give two importance’s
(b) Write product and balance the following chemical equations
- AgNO3(aq) + NaCl(aq) →
- Zn(s) + H2SO4 (aq) →
- MgCl2(aq) + AgNO3(aq) →
- ZnCO3(s) + HCl(aq) →
- Na(s) + H2O(i)→
8. (a)List down three(3) sources of Natural water
(b)Explain why water is Not used to extinguish classic E fires
(c)Give reason to Support the fact
- Water is Universal Solvent
- Oxygen is collected over water
- Oxy-hydrogen used in welding
SECTION C (30 Marks)
Answer Any two Questions
9. (a)Define molecular and Empirical formula
(b)A compound oxygen M is composed of 52.2% carbon, 13% hydrogen and the rest Oxygen. If molecular mars of M is 4
- Find empirical formula of the compound
- Find its molecular formula
10. With aid of diagram illustrate/Describe sit methods of separating mixtures
11. Most areas in Dar es Salaams have problem of water hardness which affect much of their life. As an expert explain how you can help them solve the problem
FORM THREE CHEMISTRY EXAM SERIES 117
FORM THREE CHEMISTRY EXAM SERIES 117
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM THREE-MARCH/APRIL-2022
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (15 Marks)
Answer All questions in this section.
- For each of the items (i – x) choose the correct answer from among the given alternatives and write its letter in the table provided
- Kinetic nature of matter describe
- The shape of three states of matter
- The movement of particles in the three state of matter
- The process of treating solid, liquid and gas
- Describe the meaning of matter
- Make up of matter
- Nitrogen (III) oxide has a formula
- N2 O5
- NO3
- NO2
- N2 O3
- N3O2
- A pair of the following substances from a miscible liquid
- Paraffin and water
- Paraffin and benzene
- Water and glycerin
- Water and kerosene
- Water and milk
(vi) Which one of the following sets of elements is arranged in order to increase
electro negativity starting with the least one?
- Chlorine, fluorine, nitrogen, oxygen, carbon
- Fluorine, chlorine, oxygen, nitrogen, carbon
- Carbon, nitrogen, oxygen, chlorine, fluorine
- Nitrogen, oxygen, chlorine, carbon, fluorine
- Nitrogen, oxygen,sodium, carbon, fluorine
- “Striking back” means
- Luminous flame
- Flame burning inside the barrel when the air hole is open
- Non-luminous flame
- Flame burning inside the barrel when the air hole is closed
- Excess gas
- Element Q of atomic number 15 is found in
- Group V and period 3
- Group I and period 2
- Group III and period 4
- Group IV and period 1
- Group 15 period 5
- Which of the following natural processes is not a chemical change?
- Photosynthesis
- Respiration
- Rain formation
- Corrosion of iron
- Souring of milk
- This mixture of substances can extinguish fire
- Oxygen and nitrogen
- Carbon and sand
- Carbondioxide and hydrogen
- Carbondioxide and sand
- Hydrogen and oxygen
- The physical test for oxygen is
- Support the burning splint
- Colourless, no smell and tasteless
- Produces “POP” sound
- Turns lime water blue
- Has sweet aroma
- An element ‘A’ of element configuration 2:8:3 combines with an element ‘B’of configuration 2:6. The chemical formula of the compound is
- B6 A3
- A3 B6
- A2 B3
- A3 B2
- B2A3
SECTION B.
- Match the item in list A with the responses in list B by writing the letter of the correct response in list the box below.
List A.
- Octet state
- Fossil fuels
- Rusting of iron
- Desiccators
- Solvent extraction
List B.
- Hardening of oil
- Aqueous
- Full of eight electrons in the outermost shell
- Water and kerosene
- Inert state
- Electronic configuration
- Coal, natural gas
- Used to obtain oil from groundnuts
- Potassium, sodium
- Enzymes
- For drying substance
- Condensation
- Ionic equation
- Catalyst
- Chemical change
- Physical change
SECTION B (70 Marks)
Answer all questions in this section
- (a) Define the following terms:
- Fuel ……………………………………………………………………………………………………………………………………………………
- Calorific value of a fuel ……………………………………………………………………………………………………………………………………………………
- Energy value of a fuel ……………………………………………………………………………………………………………………………………………………
(b) Give two examples of each of the following:
(i) Solid fuel ………………………………….., ……………………………..
(ii) Liquid fuel…………………………………..,……………………………...
(iii) Gaseous fuel ……………………………….., …………………………….
(c) Name four characteristics of a good fuel
(i) ………………………………………………………………………………
(ii) ………………………………………………………………………………
(iii) ……………………………………………………………………………..
(iv) ……………………………………………………………………………..
- (a) Water is said to be a compound. Verify this statement
- …………………………………………………………………………
- ………………………………………………………………………….
- ………………………………………………………………………….
- ………………………………………………………………………….
(b) Study the apparatus arranged below and answers the questions below.
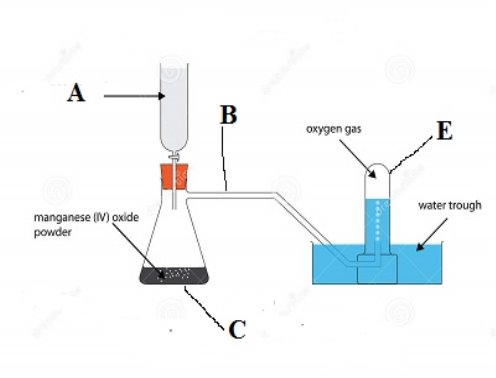
- Name the apparatus labeled
- ……………………………………………………………………
- ……………………………………………………………………
- …………………………………………………………………..
E…………………………………………………………………..
- What is the function of the apparatus labeled E? …………………………………………………………………………
- What is the role of manganese (IV) oxide (MnO2) in the above experiment? ………………………………………………………………………......
- How can you test the presence of oxygen …………………………………………………………………………..
- What was the aim of the above experiment? …………………………………………………………………………..
- Write a balance chemical equation of the reaction which occurred in apparatus C above …………………………………………………………………………
- (a) (i) Define valence ………………………………………………………………………………………………………………………………………………………………………………
(ii) Write down the valence of the following elements:
Magnesium …………………………………. Lithium ……………………………
(iii) Name the following radicals and state their valences
| Radical | Name | Valence |
| NH4+ |
|
|
| CO 32 - |
|
|
| HCO3- |
|
|
| SO32 |
|
|
| SO2 4- |
|
|
| NO 3- |
|
|
(b) Write the following compounds
(i) Aluminium oxide ………………………………………………………………
(ii) Lead (II) nitrate ……………………………………………………………..…
(iii) Ammonium carbonate …………………………………………………………
(iv) Lead (II) sulphide ……………………………………………………………..
(v) Copper (II) hydroxide …………………………………………………………
- (a) Define:
- Empirical formula ……………………………………………………………………………………………………………………………………………………
- Molecular formula ……………………………………………………………………………………………………………………………………………………
(b) A certain gaseous compound contains 82.8% of carbon and 17.2% of hydrogen by mass. The vapour density of the compound is 29. Calculate its molecular formula (C = 12, H = 1)
- (a) By giving one example define the following terms
- Suspension ……………………………………………………………………………………………………………………………………………………
- Electroplating ……………………………………………………………………………………………………………………………………………………
- Galvanizing ……………………………………………………………………………………………………………………………………………………
- Element …………………………………………………………………………………………………………………………………………………….
- Matter ……………………………………………………………………………………………………………………………………………………
(b) Give four uses of hydrogen gas
(i) ……………………………………………………………………………….
(ii) ………………………………………………………………………………
(iii) ……………………………………………………………………………..
(iv) ……………………………………………………………………………..
- (a) What do you understand by the term isotope? ………………………………………………………………………………………………………………………………………………………………………………
(b) Chlorine has two isotopes of chlorine – 35 3517 Cl, which constitutes of 75% in the mixture and chlorine – 37 3717 Cl, which constitutes of 25%. Calculate the relative atomic mass of chlorine.
(c) One of the methods of preventing iron from rusting is sacrificial protection,
why it is called so ……………………………………………………………………………………………………………………………………………………………………………………
9. (a) Give the meaning of the following terms
(i) Solution ……………………………………………………………………………..
(ii) Emulsion ……………………………………………………………………………
……………………………………………………………………………………
(iii) Potable water ………………………………………………………………………
…………………………………………………………………………………….
(b) State the modern periodic table
………………………………………………………………………………………………………………………………………………………………………………………………
(c) Name heat sources used in the laboratory
(i) ………………………………………………………………………………
(ii) ……………………………………………………………………………..
(iii) ……………………………………………………………………………
(iv) ……………………………………………………………………………
10. (a) Show how the following change in the periodic table:
(i) Electronegativity ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Ionization energy ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(iii) Atomic size ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) “Chemistry is the useless subject” Discuss the above statement with reasons
(i)……………………………………………………………………………………………
…………………………………………………………………………………………
(ii) …………………………………………………………………………………………..
…………………………………………………………………………………………..
(iii) …………………………………………………………………………………………
………………………………………………………. ………………………………..
(iv) …………………………………………………………………………………………
…………………………………………………………………………………………
11. a) What is the hardness of water?
b) Briefly explain types of hard of water.
c) State the causes of hardness of water for each type mention in (b) above.
d) Explain how you would remove the hardness of water according to its type.
e) Give three (3) advantages and three (3) disadvantages of the hard water.
12. Balance the following equations:
(i)Ca + H3PO4→ Ca3(PO4)2 + H2
- Cu + HNO3 → Cu (NO3 )2 + NO2 +H20
- SnCi2+FeC13→SnC14+FeCI
13. Give the name of the types of reaction represented by each of the following chemical equations.
- C3H8(g) +50,(0)→ 3CO2 + 4H20(1)
- 2Pb (N 03),(,)→2Pb0(,) + 4NO2 +02(g)
(iii)Zn(s)+CuS04(aq) —>ZnSO4(aq) +CU(S)
14. Complete the following equations and determine the type of chemical reaction involved in each case.
(i) Zn(s)+ H2SO4(aq)→
(i) AgN 03(aq) + NaCl(aq)→
(iii) N2(g) + H2(g) →
FORM THREE CHEMISTRY EXAM SERIES 74
FORM THREE CHEMISTRY EXAM SERIES 74
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY 1 MID TERM EXAMINATION
FORM THREE-2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- The region around the nucleus of an atom where electrons are found is called:-
- Shell (orbits or energy levels)
- Solar or Lunar eclipse
- Ozone layer
- Outer most shell.
- The following is a chemical property of oxygen.
- It burns completely when exposed and produce a “pop sound”.
- It supports combustion .
- It produces unpleasant smell
- It reacts with vapour to form carbon dioxide gas.
- _________ is one of the use of hydrogen
- it is used by mountain climbers
- it is used in painting
- it is used in manufacturing of ammonia
- it is used for breathing.
- A special catalyst used in the laboratory preparation of oxygen gas by using potassium chlorate (KClO3) is:-
- Manganese (IV) oxide
- Zinc granules
- Anhydrous copper (II) sulphate
- Gas jar
- Which of the following chemical species have the same number of electrons?
- Cl, Be and O2-
- K+, Ca2+, Cl-
- O2-, Ca2+ and Ar
- Na+, Mg2+, Be2+ and Li.
- Match each item in list A with response in list B by writing its correct letter to the number of the corresponding item in the table provided below.
| LIST A | LIST B |
|
|
| QN | I | II | III | IV | V |
| ANSWER |
|
|
|
|
|
SECTION C (70 MARKS)
- (a) use the following information about elements P,Q,R,S and T shown in the table below to answer the questions that follows.
| Element | Atomic number | Atomic mass |
| P | 8 | 16 |
| Q | 9 | 19 |
| R | 11 | 23 |
| S | 6 | 12 |
| T | 18 | 40 |
Questions:-
- Write down the electronic configuration of elements: P,Q,R,S and T.
| Element | P | Q | R | S | T |
| Electronic configuration |
|
|
|
|
|
- How many neutrons are present in element T?
______________________________________________________________________________________________________________________
(b) Write the chemical symbols for the following elements:-
(i) Magnesium________ (ii) Tin _______(iii) chromine_____ (iv) Lead______
(c ) For each of the following chemical symbols of elements write its corresponding chemical name:-
- Si______________ (ii) Cu____________ (iii) Mn_____________
- The figure below is part of the periodic table which includes the first 20 elements. Study it carefully and answer the question that follows.
| 1 |
| 18 | ||||||
| 2 | 5 |
| 8 | 10 | 12 | 14 | 16 | 19 |
| 3 | 6 |
| 9 | 11 | 13 | 15 | 17 | 20 |
| 4 | 7 | Transitional elements |
|
|
|
|
|
|
Questions:-
For each number write the chemical symbol of the corresponding element
| Number | 1 | 2 | 4 | 6 | 8 | 11 | 12 | 17 |
| Symbol |
|
|
|
|
|
|
|
|
- Write the formula which represents a compound formed between an element with atomic number 1 and element with atomic number 17. ___________
- Considering the elements with atomic number 12 and 17, which is a metal and which is a non – metal?
| Atomic number | Comment |
| 12 |
|
| 17 |
|
- Write the number which represent an element with a property of burning in oxygen to form waters________________________
- Write the number which represent and element with inert property._________
- (a) Define the following
- Empirical formula
- Molecular formula
(b) 0.7g of nitrogen combines with 1.6g of oxygen to form a nitrogen oxide. Given the molecular mass of a compound is 92.
Calculate: (i) The empirical formula
(ii)The molecular formula
(Use atomic masses N= 14, O= 16).
- (a) (i) What is water?______________________________________________
(ii Write a chemical formula for water_______________
- Outline three (3) uses of water ________________________________________________________________________________________________________________________________________________________________________
(b) (i) What is a fuel?_____________________________________________
(ii) Give two examples for each of the following categories of fuels in terms of their states of matter
Solid fuels_____________________________________________________
Liquid fuels_____________________________________________________
Gaseous fuels__________________________________________________
6. (a) Define the term a chemical equation.
(b) Write down components of a chemical equations list only three (3).
(c) Balancing the following chemical equations;
- Al(NO3)3(aq) + 3NaOH(aq) Al(OH)3(s) + NaNO3(aq)
- Ba(NO3)2(aq) + (NH4)2SO4(aq) BaSO4(s) + NH4NO3(aq)
- CaCl2(aq) + Na2CO3(aq) NaCl(aq) + CaCO3(s)
- Zn(NO3)2(aq) + Na2S(aq) NaNO3(aq) + ZnS(s)
- Na2CO3(aq) + HNO3(aq) NaNO3(aq) + H2O(l) + CO2(g)
7. (a) Give the IUPAC names for each of the following compounds:-
- Cu2O______________________(ii)Na2SO4_________________________________
- Fe2O3__________________________
(b) Calculate the oxidation number of underlined elements:-
(i) CO2- (ii) HCO3 (iii) KClO3
(c) Write the electronic configuration of Cl- and hence draw its electronic diagram.
8. Explain the meaning of each of the following types of chemical reactions and support your explanation with the help of a relevant chemical equation.
- Synthesis (combination) reaction
- Decomposition reaction
- Precipitation reaction
- Single displacement reaction
- Neutralization reaction
- Soap solution of different amount of water are tested from four different sources and produces lather observed for 30 seconds. The three groups of waster were the untreated; boiled and treated by ion exchange. The results are as follows;
| Sample | Volume of soap (cm3) used for water that was | ||
| Untreated | Boiled | Passed through ion exchanger | |
| A B C D | 12 17 26 1.6 | 1.8 17 20 1.6 | 1.8 1.7 1.8 1.6 |
Use the above results to answer the following questions;
- Which is the hardest water sample? Why?
- Which sample is like distilled water? Explain.
- Which chemical substance might be the cause of hardness in (i) Sample A (ii) Sample B?
- Write an equation for the reaction of removing hardness in sample C.
10. (i) Define the following terms;
- Soft water
- Hard water
- Permanent hardness of water
- Temporary hardness of water
(ii) Explain why the ability of temporary hard water to conduct electricity falls when the water is boiled but does not fall when temporary hardness is removed by addition of washing soda.
11. Give a brief account to the following
- Why does water note have any effect on litmus paper?
- (i) What would happen to a well stoppered bottle full of water left in a deep freezer over the night? Why does this happen?
(ii) Why is iron not usually recommended in the construction of steam pipes and boilers.
- (i) Name two compounds which may cause temporary hardness of water and two ions which largely cause permanent hardness of water.
(ii) Write one balanced chemical equation which shows how temporary hardness can be removed by boiling. Also write one balanced equation which shows how sodium carbonate can be used to remove permanent hardness of water.
12. (a) Define the following words:-
- Deliquescence
- Efflorescence
(b) Give at least three (3) uses of salts
(c ) When zinc granules and dilute sulphuric acid are reacted together, gas M is produced. The gas produced is collected by downward displacement of water. Use this information to answer the questions below:-
- Name the gas M
- How is gas M tested?
- Why is a the gas collected by down ward displacement of water?
SECTION C (15 Marks)
Answer one question in this section.
13. For each of the following write equations for the reactions that would take place.
- Action of heat on Sodium nitrate, Lead (II) nitrate and mercury (II) Nitrate.
- Action of heat on sodium carbonate and copper (II) Carbonate.
14. (a) 416g of anhydrous barium chloride where obtained when 488g of hydrated salt were heated.
Calculate the value of n is the formula BaCl2.nH2O (Ba = 137)
(b) Give the meaning of the following;
(i) An acid
(ii) Molar solution of an acid
(iii) Give three characteristics of acid.
FORM THREE CHEMISTRY EXAM SERIES 49
FORM THREE CHEMISTRY EXAM SERIES 49
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION AND LOCAL GOVERNMENT
MID TERM EXAMINATION-MARCH 2020
CHEMISTRY FORM THREE
NAME………………………………….………………..CLASS…………………………….……………TIME: 3HRS
INSTRUCTIONS:-
1.This paper consists of sections A,B and C
2.Answer all questions in all sections
3.Whenever necessary, the following constructs may be used.
- Atomic masses : O= 16, Na= 23, S=32,H=1, K=39, Al= 27,
- GMV at STP = 22.4dm3
- Avogadro’s constant = 6.02 x 1023
- 1litre = 1dm3= 1000cm3.
SECTION A (15 marks)
- Write the letter of the correct answer in the answer sheet (s) provided for each of the following questions (i) to (x).
- All domestic utensils made of iron undergo rusting when exposed to:-
- Air and fire
- Air and oil
- Air and water
- Water and oil
- When a small amount of common salt is dissolved in a glass of water the mixture formed is:-
- Heterogeneous
- Homogenous
- Immiscible
- Suspension
- A chemist should acquire all of the following skills EXCEPT:-
- Experimentation
- Observation
- Problem identification
- Surgery
- An important property of oxygen which distinguishes it from other gases is that it:-
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor support combustion
- Support combustion but does not burn
- The process of chlorination in water treatment aims at:-
- Forming suspension
- Killing micro- organisms
- Making syrup
- Removing bad odour
- One of the following is not correct about coke being a better fuel than coal as it:-
- Does not produce carbon dioxide gas
- Does not produce poisonous gas
- Has a higher heat content
- Is clean and smokeless
- Class E fire can best be extinguished by using:-
- Carbon dioxide
- Fire blanket
- Sand
- Water
- The following is a set of apparati which are used for heating:-
- Crucible, test tube, evaporating dish
- Evaporating dish, tongs, crucible
- Test tube, evaporating dish, tongs
- Tongs, crucible, test tube.
- Which of the following methods can be used to get oil from cotton seeds?
- Decantation
- Distillation
- Grinding and distillation
- Grinding followed by squeezing
- Which of the following apparati is suitable for measuring volumes of smaller quantities of liquids?
- Beaker
- Burette
- Conical flask
- Measuring cylinder
- Match each item in list A with response in list B by writing its correct letter to the number of corresponding item in the answer sheet(s) provided.
| LIST A | LIST B |
|
|
SECTION B (70 marks)
- (a) Define the term “neutralization reaction” (give one example)
(b) Write down the names and formulae of three common acids in the laboratory.
(c ) What is an indicator? Give four (4) examples of acid- base indicators.
(d) Write down the products formed when each of following pairs of compounds react:
(i) Acid and metal
(ii) Acid and metal carbonate
- (a) Complete and balance the molecular equations for the following reactions:-

(b) Write a balanced chemical equation and its corresponding ionic equation for the reaction between dilute sulphuric acid and:-
- Sodium hydroxide solution.
- Zinc granules.
N.B. show all the state symbols.
- (a) Define the terms (i) Mole (ii) Molar mass
(b) Calculate the molar mass of (i) Al2 (SO4)3 (ii) Na2 CO3 10H2O
( c) State the Avogadro’s law
(d) How many oxygen molecules are there in 20cm3 of oxygen gas at STP?
- (a) (i) Name any four heat sources in the chemistry laboratory
(ii) Name two types of flames produced by the Bunsen burner
(iii)How do the two above mentioned flames differ?
(b) Write down four (4) careers that are a result of studying chemistry.
- The diagram below represents the laboratory preparation of oxygen, study the diagram and then answer the questions which follow:-

- (i) Label the parts indicated with letters A, B, C, D in the diagram
(ii) Does Oxygen burn? Why?
- The formula of manganese (iv) oxide is MnO2 and that of hydrogen peroxide is H2O2. Which compound produces oxygen?
- (i) What is the name of the method of collection the gas?
(ii) Explain the meaning of catalyst.
(iii)How can you test for oxygen?
- Study the following part of the periodic table and then answer the questions that follows.
Note: The letters used are not the scientific symbols for the elements concerned.
|
I O | |||||||
|
| II | III | IV | V | VI | VII |
|
|
|
|
|
|
|
| N |
|
|
| K |
|
|
| Q |
| P |
| L |
|
|
|
|
|
|
|
- Identify and write down the electronic configuration for the elements K,N,P and L
- What type of bond will exist in a compound formed when Q combines with L? write the chemical formula for the compound formed and list two chemical properties of the compound formed.
9. (a) Write the chemical symbols for beryllium, boron, neon, nitrogen and phosphorus.
(b) Why some of the elements in 9(a) are assigned symbols with only one letter while others bear symbols with two letters?
10. (a) Give three advantages of using chemical equations over word equations.
(b) You are provided with a compound composed of 22.2% zinc, 11.6% sulphur, 22.3% oxygen, and the rest percentage is water of crystallization. Calculate the molecular formula of the compound if its molecular mass is 283
11. (a) Which ways are the fossil fuels detrimental to the environment? Give four points.
(b) Briefly explain how biogas is produced by using domestic waste.
12. (a) Write the IUPAC names of the following compounds
- NaClO3
- K1O3
- NaH
- Ca(NO3)
- NO
(b) Write the chemical formula of the following compounds
(i) Lead (II) chloride
(ii) Aluminium hydride
(iii) Sodium hydrogen phosphate (v)
(iv) Sulphur oxide (VI)
(v) Ammonium chloride
SECTION C (15 Marks)
Answer one (1) question from this section.
13. (a) Differentiate between basic salt and acidic salt.
(b) Categorize the following salts;
i. PbSO4 ii. MgHSO4 iii. Zn(OH)Cl iv. MgHCO3
v. NH4HSO4 vi. Ba(NO3)2
| Salts | Category |
|
|
|
(c) 1.16g of magnesium was allowed to react with excess dilute sulphuric acid. What
volume of Hydrogen gas at STP was liberated.
14. (a) Define the following;
- Molarity
- Standard solution
- Equivalent point of titration
- End point
(b) On titration 20cm3 of a solution containing 2.65g per 500cm3 of A2CO3, exactly
react with 20cm3 of 0.1M hydrochloric acid solution. Write balanced equation;
- Molarity of A2CO3
- Molar mass of A2CO3
- Atomic mass of A
- Identify element A
FORM THREE CHEMISTRY EXAM SERIES 3
FORM THREE CHEMISTRY EXAM SERIES 3
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







