FORM ONE CHEMISTRY EXAM SERIES 230
FORM ONE CHEMISTRY EXAM SERIES 230
THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM ONE ANNUAL EXAMINATION
CHEMISTRY
TIME: 3 HOURS NOVEMBER 2024
Instructions
1. This paper consists of sections A, B and C with a total of ten (10) questions.
2. Answer all questions. All answers must be written in the spaces provided.
3. Section A and C carry fifteen (15) marks each, Section B carries seventy (70)
marks.
4. All writing must be in blue or black ink except drawings which must be in pencil.
5. All communication devices, calculators and any unauthorized materials are not
allowed in the Mock Assessment room.
6. Write your Mock Assessment Number at the top right corner of every page.
7. The following atomic masses may be used: H = 1, C = 12, O = 16, Na = 23,
S = 32, Ca = 40.
SECTION A (15MARKS)
Answer all questions in this section.
1. For each of the following (i)-(x) choose correct answer from alternative given
i. One of the following apparatus is used to measure a fixed volume of liquids
A. Pipette
B. Burette
C. Measuring cylinder
D. Beaker
ii. Coloured substance can be separated through process called
A. Filtration
B. Chromatography
C. Distillation
D. Sublimation
iii. Which of the groups of substance represents flammable liquid
A. Petrol, Pesticides, Hydrogen
B. Petrol, Sulphuric acid, Methylated spirit
C. Methylated spirit, Petrol, Kerosine
D. Kerosine, Diesel, Hot water
iv. Acid changes colour litmus paper from
A. Blue to Yellow
B. Red to Blue
C. Red to pink
D. Blue to Red
v. Chemistry is a scientific activity because
A. Chemistry is studied in school
B. Chemistry knowledge is acquired through observation experimentation and logical reasoning
C. It is an interesting subject
D. It involves the study of non-living things
vi. On her first experiment, Lilian Dissolved sulphuric acid in water and heat was evolved. In her second experiment she dissolved sugar in water and no heat was evolved or absorbed. She conclude that;
A. Her first experiment was physical change
B. Both Of her experiment were physical change
C. Her second experiment was a chemical change
D. Her first experiment was a chemical change
vii. Loose or floppy clothing is not allowing in the laboratory, why
A. Movement has to be fast
B. It will get wet when water splashes
C. It may catch fire or cause one to fall
D. It cause poor ventilation in the body
viii. Which of the following is not a component of First Aid Kit
A. Googles
B. Gloves
C. Dropper
D. Pair of scissors
ix. If you want Bunsen Burner Flame to have the same colour on the candle flame you must
A. Have air hole completely open
B. Turn on a large supply of gas
C. Have the air hole completely closed
D. Have more candle burning
x. A rapid chemical reaction that release energy in form of light and noticeable heat is called
A. Ignition
B. Reactant
C. Combustion
D. Heating
2. Match the mixture in List A with the corresponding method of separation in List B by writing the letter of correct answer below item number.
| LIST A | LIST B |
| i. Ethanol from water ii. Salt from sea water iii. Rice from husk iv. Oil in sun flower v. Erythrocycles from blood | A. Chromatography B. Filtration C. Solvent extraction D. Fractional Distillation E. Decantation F. Layer separation G. Centrifugation H. Winnowing I. Simple distillation J. Evaporatio |
SECTION B
Answer all question
3. a) Is air a mixture or a compound. Give four reasons
b) A form two student dipped a clean in rod into a cold distilled water in a test-tube and left it for 2 days
i. State what will happen to iron after two days.
ii. Explain the observation if the rod is replaced by a painted nail in the same test tube and left there for 2 days
iii. Explain the observation if cold distilled water will be replaced by a mixture of hot water an oil
c) Explain any two methods that can be used to prevent iron from rusting by giving vivid example
4. a) Differentiate
i. Homogeneous mixture from heterogeneous mixture
ii. Miscible from immiscible liquids
b) Saypalm was in kitchen frying eggs container A and by the same time he submitted solid Iodine in other container which was labelled B
i. What changes were encounted by two substance mention two
ii. State the difference which exists between changes in (a) (i) by providing three points
5. a) Identify types of change involved in each of the following
i. Respiration
ii. Sublimation
iii. Combustion
iv. Distillation
b) Write the chemical symbol of the following
i. Potassium
ii. Sodium
iii. Mercury
iv. Gold
c) Write the most suitable method of separating the following mixture
i. Air
ii. Kerosene and water
iii. Iodine and sand
iv. Syrup
6. a) What do you understand by the term fire
b) Mushi Burned a piece of wood and result were energy and light
i. Name chemical reaction involved
ii. What is the name of given to piece of wood in this reaction
iii. With one example write three areas where chemical reaction in (b) (i) above is applied
7. a) Laboratory technician planned to label warning signs on container found in laboratory. Suggest the name of warning sign he could label on the container with the following;
i. Rat poison
ii. Methylated spirit
iii. Hydrogen peroxide
iv. Concentrated sulphuric acid
v. Cooking gas
b) Briefly explain how you can help an individual who has fainted
8. a) Define a flame
b) Identify factors (2) to consider when choosing a flame
c) Write five properties of luminous flame
9. a) Justine is not interested in studying chemistry. Briefly explain to him five reasons why he should study chemistry.
b) With examples indicate five areas where chemistry is applied.
SECTION C 15 marks
10. a) Differentiate between
i. An element and an atom
ii. A compound and mixture
b) What should you do immediately if;
i. A piece of paper of broken beaker cuts your figure
ii. Chemical splash on your face
iii. Your shirt has caught fire
iv. Your fellow student swallow unknown chemical substance thinking that it was water
c) State the uses of the following in first Aid
i. The pair of scissors
ii. Petroleum jelly
iii. Whistle
iv. Sterile gauze
FORM ONE CHEMISTRY EXAM SERIES 187
FORM ONE CHEMISTRY EXAM SERIES 187
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
ANNUAL EXAMS – NOVEMBER – 2023
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A. (15 MARKS)
- For each of the item number (i) – (x) Choose the correct answer from given alternatives.
- A simple proof that chemical reaction takes place in our bodies is that;
- We eat balanced diet
- Doctor tells us so in hospital
- We occasionally fall sick
- The food we take is quite different from waste that comes from our bodies
- There is no proof
- What change of state is involved when drying wet clothes?
- Gas to solid
- Liquid to gas
- Gas to liquid
- Solid to gas
- Liquid to solid
- Which is suitable alternative heat source to be used in absence of Bunsen burner?
- Kerosene stove and torch
- Torch and spirit burner
- Kerosene, stone and spirit burner
- Fire wood and torch
- Torch and candle
- Sodium is abbreviated as Na but both letters are exclusive in the word sodium, this is because,
- The Latinized as naterite
- Comes from the world Natural
- The Latin name of sodium is Natritium
- It is derived from two letters of its English name
- It is derived from English name
- Fire triangle is the component required for a fire to start. This includes
- Heat, Fuel, Energy
- Heat, fuel, and air
- Heat, water, and Energy
- Heat, Iron and light
- Heat, light and Energy
- Which of the following is unlikely to produce soot?
- Complete combustion
- Incomplete combustion
- Limited supply of air burning
- Luminous flame
- Air holes open
- Mixture of cooking Oil and water can be termed as
- Suspension
- Solution
- Emulsion
- Saturation
- solvent
- the process of coating iron or steel with zinc is known as
- Zinc painting
- Tin plating
- Alloying
- Electroplating
- Galvanizing
- Which among the following lists contain the items transported that are produced as a result of application of chemistry?
- Dyes, tyres and fuel
- Coolant, fuel and Lubricants
- Pants, lubricants and pesticide
- Lubricant, tyres, and drugs
- Coolant plant Dyes
- Which of the following group of substances represents flammable liquids
- Petrol, pesticide and hydrogen
- Petrol sulphuric acid and methylated spirit
- Methylated spirit, Petrol and Kerosene
- Kerosene, Diesel and hot water.
- Oxygen, Hydrogen peroxide, Petrol
- Match the materials in LIST A with correct method of preventing it from rusting in LIST B by writing the letter of correct answer below item number in table provided.
| LIST A | LIST B |
|
|
SECTION B. (70 Marks)
Answer all questions in this section
- (a)Briefly explain two conditions necessary for a substance to be called matter
(b)Differentiate solution from suspension using four points.
- (a) Give one (1) reason to support the following statements.
- A luminous flame in said to be safer and less likely to cause accident in the laboratory.
- Chemical which are not correctly labelled should not be used in laboratory
- The component of a mixture of water and cooking oil are separated using layer separation method.
(b) Give two examples of apparatus that are made of
- Porcelain
- Plastic material
- Glass material
- Iron materials
- (a) Describe four importance of first Aid to an accident victim.
(b) Mary dipped a clean iron rod into cold distilled water in a test tube and left it four 2days. Explain Observation after two days.
- State what will happen to the iron rod after 2 days
- Outline three things which cause process in (b) above
- How could these student Prevent the process above despite of the conditions state. Give two method
- (a)What do you understand by the world fire?
(b) Josephine burnt a piece of wood and the results were energy and light
- Name the chemical reaction involved
- What is the name given to a piece of wood in this reaction?
- With One example write three areas where chemical reactions in (b) (i) above is applied
- (a)Identify types of change involved in each of the following, weather physical or chemical change
- Respiration
- Sublimation
- Combustion
- Distillation
(b) Write the chemical symbols of the following elements.
- Potassium
- Sodium
- Mercury
- Gold
- (a)Halima needed to measure the volume of water in a chemistry laboratory. Which apparatuses. Should be used (3 apparatuses)
(b)Name four accidents that needs first Aid procedures in Laboratory
(c) With reason state one method suitable to separate each of following
- Ethanol and water
- Cooking and water
- Ammonium chloride and sand
- (a)Most of famers in rural areas were interested to know how the knowledge of chemistry is necessary in Agriculture activities. Assume you are a chemist; educate these farmers on application of chemistry in agriculture by giving five points.
(b)Why is Non-luminous flame better than luminous flame in heating?
SECTION C (15 Marks)
- Scientific procedures are steps used by scientists when finding answer to scientific problems. Write the steps which correspond to each of the following
- Kelvin was not feeling well. She went to see a medical doctor at Mawenzi hospital
- The Doctor asked Kelvin Several questions on how he was felling
- The doctor Ordered Kelvin body temperature, blood and Urine sample for Observation in the laboratory.
- The laboratory technician diagnosed Malaria parasite in Kelvin Blood.
- The Doctor confirmed that Kelvin had Malaria and prescribed medicine for him.
(b)Why is scientific procedure? Give two points
(c) State three areas where scientific procedures are applied
FORM ONE CHEMISTRY EXAM SERIES 148
FORM ONE CHEMISTRY EXAM SERIES 148
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
TIME: 2 HOURS NOVEMBER 2022
INSTRUCTIONS
1. This paper Consist of sections A, B and C with total of 10 questions
2. Answer all questions in both sections
3. All writings should be in blue/black ink
4. All diagrams should be drawn in pencil
5. Write your assessment number at the top right corner
SECTION A (15Marks)
1. For each of the items (i)-(x), choose the correct answer from among the given alternative and write its letter in the box provide.
- The welders prefers to use non luminous flame for their work simply because
(A)It is available (B) it is easy to transport
(C) produce very hot flame (D) can be made by kerosene
- Spatula in the laboratory is used for scoping what types of substances?
(A)Liquid and gases (B) solids and liquids
(C) Powdery and gases (D) Solids and powdery
- Why oxygen as one of the components of air Is unique?
(A)It has ability to burn (B) it support combustion
(C) it is diatonic gas (D) combine with carbon dioxide
- Why it is necessary to boil drinking water?
(A) To make it tasteless (B) To remove impurities
(C) to kill micro-organism (D) To make it taste less
- How do chemists refer to a mixture of milk and water?
(A)Miscible solution (B) Suspension (C) Immiscible solution (D) Emulsion
- How can one prevent rusting in fragile instruments like camera?
(A)By galvanization (B) By using silica gel (C)By using oil (D) By using ethanol
- Which of the following is not man-made product of applying chemistry?
(B) Sugar (B) Fertilizer (C) Milk (D) Vaccines
- li>Spatula in the laboratory is used for scoping what types of substances? (A)Liquid and gases (B) solids and liquids
(C) Powdery and gases (D) Solids and powdery
- Fractional distillation is possible if the mixture constituents differ in _______
A. Boiling points
B. Melting points
C. Vaporing points
D. Freezing points
- Which of the following is NOT among the composition of air?
A. Noble gases
B. Hydrogen
C. Carbon dioxide
D. Nitrogen
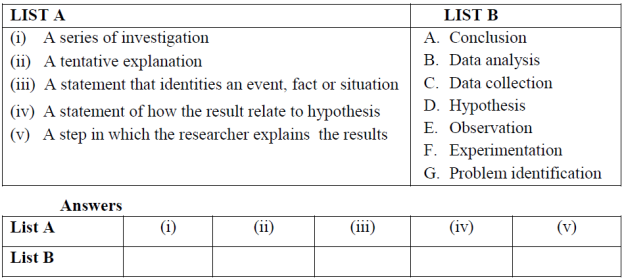
2. Match the descriptions in List Awith their corresponding answer in List Bby writing the letter of the correct response beside the item number in the answer booklets (s) provided.
SECTION B: (70 Marks)
Answer all questions in this section
3. (a) Suggest the best method of separating the following mixtures:
i. Ethanol and water__________________________________________
ii. Sodium chloride and water___________________________________
iii. Green solution form leaves___________________________________
iv. Sand from ice_____________________________________________
v. Iron feelings and calcium carbonate powder____________________
b) i. Why is zinc used as a coat for iron and not vice versa?
ii) Is water a mixture or a compound? Give two reasons to support your answer.
4. (a) Mrs. Msema kweli was addressing the villagers about use of water for economic values. What 4 values will she tell the villagers
(b) Is an air a mixture or a compound? Give 4 reasons
5. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv. Sterile gauze
6. (a)Distinguish the following substances.
- Homogenous mixture from heterogeneous mixture.
- Miscible from immiscible liquids.
- Saturated from unsaturated solution.
(b)Give two chemical tests for pure water
7. (a) Water exists in three states of matter with two examples, mention three states
of matter.
(b) The figure below shows the relationships among the three states of matter. Name the process involved in A, B, C, D, Eand F.
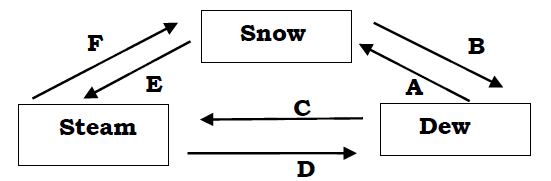
ii. Mention one (1) Chemical test for water
8. (a) Briefly explain the uses of the following;
- Oxygen gas
- Carbon dioxide gas
- Nitrogen gas.
(b) Complete the following table
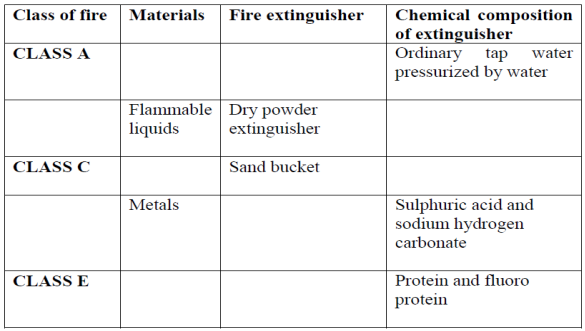
9. (a) What should you do immediately if:
(i) The piece of broken beaker cuts your finger
(ii) Chemicals splash on your face
(iii) Your shirt has caught fire
(iv) Your fellow student swallows unknown chemical substance thinking that it was water
(b) Why do you think first aid is an important thing? Give two points:
SECTION B: 15 MARKS
10. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
FORM ONE CHEMISTRY EXAM SERIES 116
FORM ONE CHEMISTRY EXAM SERIES 116
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY ANNUAL EXAMINATION
FORM ONE-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
SECTION A – (20 MARKS)
1. For each of the item (i)-(x) choose the correct answer from the choices given and write the corresponding letter in the table provided.
- Air entering the Bunsen burner is controlled by ___________(A) metal ring (B) air hole (C) metal jet (D) air ring
- Which of the following apparatus is used to measure a fixed volume of liquid? (a) burette (b) measuring cylinder (c) pipette(d) dropper
- A branch of science which deals with the study of matter and its properties is called ________ (a) meteology (b) astronomy (c) physics (d) chemistry
- Petrol is an example of _________ (a) corrosive substance (b) irritant substance (c) flammable substance (d) toxic substance
- First aid is provided to: (a) any person who is sick (b) an accident victim (c) an ill person (d) doctors and nurses
- A chemist should acquire the following skills except (a) experimentation (b) observation (c) problem identification (d) surgery
- One of the following is not laboratory rules. (a) heat flammable chemicals over open flame (b) turn off all burners when not in use (c) always wears protectors during experiment (d) never use broken glassware during experiment
- The chemical symbol lead is _________ (a)Nw (b) Pb (c) Ar (d) Sd
- Chemical spread on crops to destroy pests are called __________ (a) insecticide (b) pesticides (c) fertilizers (d) weed killers
- A pharmacist is a person who deals with ____________ a) chemical processing industries (b) medicine (c) laboratory experiment (d) agriculture
| I | ii | Iii | Iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
2. a) Match each item in list A with the correct response in list B by writing its letter below the number of the corresponding item in the table provided
| LIST A | LIST B |
| A. hospital B. Vaccines C. Luminous flame D. deposition E. sublimation F. non luminous flame G. experimentation H. formulation hypothesis I. laboratory
|
| List A | I | Ii | iii | iv | v |
| List B |
|
|
|
|
|
b) fill the blanks provided
- Chemical used to kill insects are called ______________
- Are the people who study chemistry in ancient times ____________
- __________ is the study of nature
- The study of chemistry prepares people for different skills we need in our daily life such as _____________
- ____________ is anything which has mass and occupies a space
SECTION B (80 MARKS)
3 (a) Define chemistry __________________________________________________________________________________________________________________________
(b) Name two product in each of the following field made by application of chemistry
| Field | products |
| i) agriculture | a) |
|
| b) |
| ii) food and beverage industry | a) |
|
| b) |
|
|
|
c) mention four importance of studying chemistry
- ___________________________________________________________________________________
- __________________________________________________________________________________
- _________________________________________________________________________________
- _____________________________________________________________________________________
4. a) Define the following terms
- First aid _____________________________________________________________________________________________________
- First aid kit ___________________________________________________________________________________________________________________________________________________
- Fainting ____________________________________________________________________________________________________________________________________________
(b) Mention four importance of first aid to a victim
- _________________________________________________________
- _________________________________________________________
- ____________________________________________________________
- ________________________________________________________________
5. a) Draw and give one function of each of the following apparatus
| Name of apparatus | Drawing | functions |
| i) conical flask |
|
|
| ii) pipette |
|
|
b) mention four components of first kit and state their uses
- ________________________________________________________________________________
- ________________________________________________________________________________
- __________________________________________________________________________________
- ________________________________________________________________________
6. a) Mention three (3) state of matter and give three (3) examples of each state
- ________________________________________________________
- _______________________________________________________
- __________________________________________________________
b) what type of change are these
- Decaying of teeth _______________________________________________________
- Burning of charcoal _____________________________________________________
- Drying of wet clothes ____________________________________________________-
- Grinding piece of chalks ______________________________________________
- Ripens of fruits _______________________________________________________
- Sour milk ____________________________________________________________________
7. a) what is the differences between physical change and chemical change (4 points)
| PHYSICAL CHANGE | CHEMICAL CHANGE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b) The figure below shows the relationship among three states of matters: Name the process involved in A, B, C, D, E and F
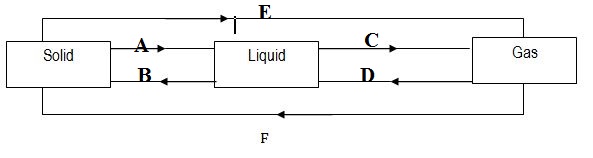
- Is____________________________________________
- Is_________________________________________________
- Is __________________________________________________
- Is_____________________________________________________
- Is_________________________________________________________
- Is __________________________________________________________-
8. a) i) what is flame? _______________________________________
ii) mention two types of flame produced by Bunsen burner
- ____________________________________________________________-
- ___________________________________________________________
b) Give four differences between the flames you have mentioned in 8 a (i) above
| i) |
|
| ii) |
|
| iii) |
|
| iv) |
|
9. What do you understand about the following terms
- Mixture____________________________________________________________________________________________________________________
- Solute _________________________________________________________________________________
- Solvent _____________________________________________________________________________________________________________________________
- Solution _____________________________________________________________________________
- Compound _____________________________________________________________________________________________________
10. i) Define an element______________________________________________________________________________________________________-
ii) write a chemical symbol for each of the following elements
- Iron _________________________________________________________________
- Copper __________________________________________________
- Silver _______________________________________________________
- Mercury _______________________________________________________
iii) write the name of the following element for each chemical symbols
- K_________________________-
- Ne__________________________
- Ca_______________________
- Pb __________________________-
FORM ONE CHEMISTRY EXAM SERIES 72
FORM ONE CHEMISTRY EXAM SERIES 72
PRESIDENT'S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ANNUAL EXAMINATION
CHEMISTRYFORM ONE
NAME………………………………………..CLASS……………………………TIME: 2HRS
INSTRUCTIONS:-
-This paper consists of three sections- A, AND B
-Answer all questions in section A and B.
SECTION A: 15 MARKS
- Choose the most correct answer from among of the given alternatives and write its letter in the space provided;
- The following skills acquired through studying chemistry except;
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Chemistry is a scientific activity because;
- Chemistry is studied in schools
- Chemistry is interesting subject studied through data collection and analysis.
- Knowledge of chemistry is acquired through observation, experimentation and logical reasoning.
- It involves study of living and non living things.
- The following are laboratory rules except;
- Turn off water and gas tap after use
- Test any unlabelled substance before using it in the laboratory.
- Put off flames which are not in use
- Remove obstacles from gang ways.
- Splints are among of the first aid kit equipments which of the following sentences set describes its use;
- Used to pick up and holding things
- Used for cutting bandages or pieces of clothes
- Used to support broken bones and is tied using bandages.
- Used for holding pieces of bandages or cloth together.
- The Bunsen burner is likely to contain soot when;
- The air hole is closed
- The air hole is open but not fully
- The air holes of Bunsen burner are completely open.
- The burner is not raised
- The gas supply is high
- Which of the following takes place in experimental stage?
- Rejection of hypothesis
- Acceptance of hypothesis
- Concluding the scientific findings
- Carry out investigations
- In scientific procedure, what would be the next step to follow after experiments?
- Data interpretation
- Conclusion
- Observation
- Investigation
- In her first experiment, Mwajuma dissolved sulphuric acid in water and heat was evolved. In her second experiment he dissolved sugar in water and no heat was evolved or absorbed she conducted that;
- Her first experiment was physical change
- Both of her experiments were physical change
- Her second experiment was chemical change
- Her first experiment was chemical change
- In most general sense the word combustion means …………….
- Burning substance in oxygen or air.
- Burning compound of carbon in air.
- Burning any fuel in air or oxygen
- By chemical combination accompanied by light and heat in which or more reactants is gaseous
- 10 grams of copper powder were placed initiation tube containing air. The tube was very strongly for half an hour. At the end experiment;
- Copper increased in mass in changed colour
- Copper melted into liquid copper form.
- The total mass of tube and its content decreased.
- Water vapour appeared in the tube.
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
- Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
All materials used for preventing rusting aim at preventing water and oxygen from reaching the iron.Write T for correct statement and F for incorrect statement;
- Accidents always happen in the laboratory.
- All experiments must be done in the fume cupboards.
- Rusting cannot cause corrosive to some articles made up iron or steel.
- Desiccator is an apparatus used to dry solid chemicals.
SECTION B 80 MARKS
(a) Define the following terms;z
- Combustion ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
- Combustible material ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Label the parts marked A, B, C, D, E and F in the diagram below;
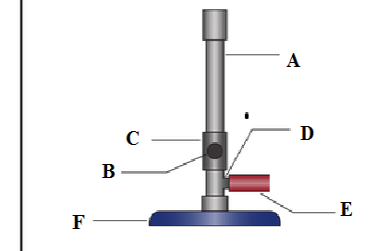
- ………………………………………..
- ………………………………………..
- ……………………………………….
- ………………………………………..
- ………………………………………..
- ………………………………………..
(c) What is the importance of parts labelled?
B ………………………………………………………………………………………
C ………………………………………………………………………………………
D ……………………………………………………………………………………….
- Fill the suitable word in the space provided;
(a)Bronze is an alloy formed by mixing ……………… and ……………………….
(b)Steel is an alloy formed by mixing ………………… and ……………………….
(c)…………………… is a solution in which like solvent used is not water.
(d)…………………….. is used to stir and mix substance uniformly.
- (a) Define the following terms and give one example in each case;
- A chemical symbol ……………………………………………………………………………………………………………………………………………………………………………………
- An element ……………………………………………………………………………………………………………………………………………………………………………………
- A compound ……………………………………………………………………………………………………………………………………………………………………………………
- Suspension ……………………………………………………………………………………………………………………………………………………………………………………
(b) Write names of elements represented by the following symbols
i.Au ……………………………………
ii. Ag …………………………………….
iii. Hg ……………………………………..
iv. P ………………………………………..
v. Sn …………………………………………
- Draw apparatus which are used to perform the following process in the laboratory;
| Process | Apparatus diagram |
| (a)It is used to measure accurate 20/25cm3 of liquid. | |
| (b)A flat apparatus at the base used to hold volume of liquid during experiment. | |
| © Wire placed on top of tripod stand. | |
| (d)Used for scooping small quantities of powder or crystalline chemicals. | |
| (e)Used for holding heating and mixing liquid – it measures estimate volume of liquids. |
- (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
i)………………………………………………………………………………………………………………………………………………………………………………
ii)………………………………………………………………………………………………………………………………………………………………………………
iii)………………………………………………………………………………………………………………………………………………………………………………
iv)………………………………………………………………………………………………………………………………………………………………………………
v)………………………………………………………………………………………………………………………………………………………………………………
(b) Distinguish between the following;
i.Hypothesis against fact ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii. Deposition against sublimation ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Point out any 6 differences between metals and non – metals ;
| Metals | Non metals |
- (a) Briefly explain any five methods of preventing rusting.
i)………………………………………………………………………………………………………………………………………………………………………………
ii)………………………………………………………………………………………………………………………………………………………………………………
iii)………………………………………………………………………………………………………………………………………………………………………………
iv)………………………………………………………………………………………………………………………………………………………………………………
v)………………………………………………………………………………………………………………………………………………………………………………
(b) A certain pink coloured compound is heated to form blue colour. When water is
added to sample , it changed back to pink.
i. What change is demonstrated by the compound …………………………………....
ii. Explain the reasons for your choice.
…………………………………………………………………………………………………………………………………………………………………………………………...….
(c) State the type of changes demonstrated by the following phenomenon;
- Burning of firewood ……………………………………………………….
- Rotting of meat …………………………………………………………….
- Heating frying pan …………………………………………………………..
- Melting of butterfat …………………………………………………………
- Change of cloud to rain ……………………………………………………..
- Heating of iron rod …………………………………………………………..
- Your provided with the diagrams below, study it carefully and answer questions that follows;
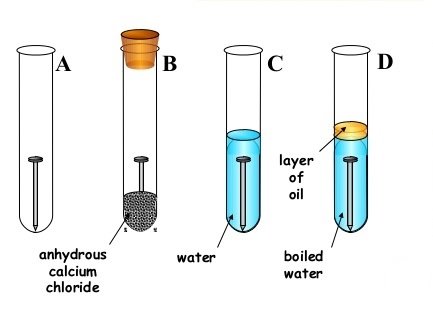
(a)State short and clear description about what will be observed in each test tube A – D after 2 or 3 days;
- Test tube A. ………………………………………………….
- Test tube B …………………………………………………..
- Test tube C. …………………………………………………..
- Test tube D. …………………………………………………..
(b)Why the water in the test tube D boiled the covered with oil. ……………………………………………………………………………………………………………………………………………………………………………………
(c)What is the function of calcium chloride in test tube B? …………………………………………………………………………………………………………………………………………………………………………………….
(d)What conclusion can you draw from your experiment? …………………………………………………………………………………………
(e)(i) Give two similarities between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Give two differences between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
FORM ONE CHEMISTRY EXAM SERIES 26
FORM ONE CHEMISTRY EXAM SERIES 26
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







