THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
132/1 CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 Hours Year: 2016
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.;
2. Answer all questions in section A and two (2) questions from section B.
;3. Each question carries ten (10) marks in section A and fifteen (15) marks in section B.
;4. Mathematical tables and non-programmable calculators may be used.;
5. Cellular phones and any unauthorised materials are not allowed in the examination room.;
6. Write your Examination Number on every page of your answer booklet(s).
7.;;For calculations, you may use the following
Rydberg constant, RH;=;1.09678x 107m-1
Gas constant, R = 8.31JmoI-;1;K;-I;or 0.0082 atm mol-1;K;-I;dm3
GMV;;at s.t.p.;;;;22.4dm3
1 litre = 1 dm3;=1000cm3
At STP: Temperature = 273K, Pressure = 760mmHg
Planck constant, h=6.63x 10;-34;Js
Velocity of light, C = 3.0 x 108;m/s
Atomic masses H = 1, C = 12, N = 14, O-16, Na-23
;
;
SECTION A
1.;;;(a) State the following:
(i);;;;;;Postulates of Bohr's atomic model.
(ii);;;Shortcomings of the Bohr's atomic model
View Ans
(b);;;;;;An electromagnetic radiation of wavelength 2420 A is sufficient to ionize the sodium atom. Calculate the ionization energy of sodium atom in kJmol-1.
View Ans
(c);;;;;;Calculate the wave number of the longest wavelength transition in Balmer series of atomic hydrogen.
View Ans
2.;;;;;;;(a) Define the following:
(i);;;;;;;;principle quantum number
(ii);;;;;;;azimuthal quantum number
View Ans
(b);;;;;;Given the quantum number, n = 3. Answer the following questions:
(i);;;;;;;;List all possible orbitals present in this quantum energy.
(ii);;;;;;;Write possible values of ml and ms for this quantum number.
View Ans
(c);;;;;;The mass spectrum of an elements enables the relative abundance ofeach isotope of the element to be determined. Data relating to mass spectrum of an element X whose atomic number is 35 appear as indicated in the table below. Study the data and answer the question that follows:
| Mass number of isotopes | Relative abundance |
| 79 | 5.5% |
| 81 | 49.5% |
;(i);;;;;;;;Define the term isotope
(ii);;;;;;;Write the convention symbols for the two isotopes of element X.
(iii);;;;;Calculate the relative atomic mass of X to three significant figures.
View Ans
3.;;;(a) Give reasons for the following observations
(i);;;;;Both sodium and hydrogen occur in group IA of the periodic table, yet the melting point of sodium chloride is 8000C while that of HCI is - 1140C.
(ii);;;;;Sodium chloride is soluble in water but not in benzene.
(iii);;;;;Although both oxygen and sulphur are in the same group of the periodic table, the hydride of oxygen (H20) is a liquid but he hydride of sulphur (H2S) is a gas at room temperature.
View Ans
(b) (i) Study the following compounds: hydrogen sulphide (H2S), ammonia (NH3), hydrogen fluoride (HF), chloroform (CHC13) and ethanoic acid (CH3COOH). With reasons, briefly describe the compounds which contain and those which do not contain hydrogen bond.
(ii) Briefly explain why dimethyl ether is more volatile than ethanol although their molecular weights are the same.
View Ans
4.;;;(a) State why was it necessary to modify the ideal gas equation and show how the modified equation looks like. Define all symbols in the equation.
View Ans
(b);;;Briefly explain why beyond certain temperatures gases cannot be liquefied
View Ans
(c);;One mole of diethyl ether occupies 1.5 litres at 2270;C. Calculate the pressure if the Van der Waals constants for diethyl ether are: a 17.38atm.litre2mol-1 and b = 0.1341itre mol-I .
View Ans
5.;;;;;(a) Define the following terms with reference to gases:
(i);;;;critical temperature
(ii);;;critical volume
(iii)critical pressure
View Ans
(b);;;;;From ideal gas equation, derive the relationship between density of a gas in grams per dm;3, the gas pressure in atmospheres, the temperature (T) in kelvin, the relative molecular mass of a gas (Mr) and the gas constant R.
View Ans
(c);A certain dry gas is composed of 21% by volume of oxygen, 1% of argon and 78% of nitrogen. Find its density in gdm-3 at 200;C and 98.65kNm-2 pressure.
View Ans
6.;;;(a) Give two differences between osmosis and diffusion.
View Ans
(b);;;;;When 15g of glucose (C6H1206) was dissolved in 50g of a certain solvent with a relative molecular mass of 180g, the freezing point was depressed by 8.0;0;C. Using these data, calculate the freezing point depression constants, for the solvent.
View Ans
(c);An aqueous solution of sugar containing 19.15g of sugar per drn3;has osmotic pressure of 136,300Nm-Z at 20;0;C. Calculate the relative molecular mass of sugar.
View Ans
SECTION B
7. (a) Give a brief molecular explanation of positive and negative deviations from Rapult's law for non-ideal binary solutions.
View Ans
(b);;;;;What vapour pressure lowering difference(s), if any, would you expect for 1M aqueous solutions of (i) CalCl2, (ii) 1<Br (iii) Na3P04? Justify your answer.
View Ans
(c);;;;;Benzene (C6H6) and toluene (C6H5CH3) form an ideal solution. At 333K, the vapour pressure of pure benzene is 53.3kPa while that of pure toluene is 26.7kPa. If a solution is prepared by mixing two moles benzene and three moles of toluene;
(i);;;;;;Find the partial pressure of each component in the vapour phase in equilibrium with this solution at 333.
(ii);;;Calculate the total vapour pressure of the solution.
(iii);Explain which substance will be collected from the top of the distillation column, if a mixture of benzene and toluene is distilled.
View Ans
8.;;;;(a) Pure ethanol (C2H50H) boils at 78.3;0C and at pressure of 760mmHg. When 2.51g of an organic compound M(Mwt =146g) is dissolved in 100g of ethanol, the solution boils at 85.90C and 760mmHg.
(i) Explain why the boiling point of ethanol was raised. (ii) Calculate the molar boiling point, Kb for ethanol.
View Ans
(b);;;;;;A solution was prepared by dissolving 2.40g of biphenyl (C12H10) in 75.00g of benzene. Calculate the boiling point of the solution given that Kb = 2.53;0C/m; Kf =5.12;OC/m; boiling point of pure benzene = 80.1oc and freezing point of pure benzene is 5.50C.
View Ans
9.;;;(a) (i) Briefly explain the dynamic nature of equilibrium reaction.
(ii) Use hydrogen (H2) and iodine (12) gases which produce hydrogen iodide (HI) gas to illustrate the point mentioned in (a)(i).
View Ans
(b);;;;;;(i) Mention the four common stresses explained by Le Chateliers' principle to; ;help maximize the yield of ammonia gas in the Haber process.
;help maximize the yield of ammonia gas in the Haber process.
(ii) The equation for production of ammonia gas is as follows:
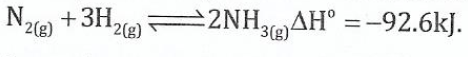
From the given equation, explain how maximum yield of ammonia can be achieved.
View Ans
10.;;;;(a) State the following:
(i);;;;;;Heat of reaction
(ii);;;;Exothermic reaction
(iii);;Endothermic reaction
View Ans
(b);;;;;;1.5g of ammonium nitrate (NH N03) was added to 35.0g of water in a plastic beaker and stirred until the salt dissolved. The temperature of the solution dropped from 22.70C to 19.40C. Basing on the given information respond to the following equations:
(i);;;;;;Is the process endothermic or exothermic? Explain.
(ii);;;Calculate the heat of solution of NH4N03 in kJ/moI., given that specific heat capacity of water = 4.184J/g0C
View Ans
SECTION C
11.;;Briefly explain the following terms and give an example of family of organic compounds in each case:
(i);;;;;;;;Hydrocarbon
(ii);;;;;;Saturated hydrocarbon
(iii);;;;Unsaturated hydrocarbon
View Ans
(b);;;;;;10cm3;of a gaseous hydrocarbon Q required 45cm3;of oxygen for complete combustion. Q reacts with I mole of bromine gas to form a brominated compound of relative molecular mass of 200.02 which contains 79.2% bromine.
(i);;;;;;;;Determine the molecular formula of Q. (ii) Give the structural formula of Q.
View Ans
12.;;Define the following:
(i);;;isomers
(ii);;;isomerism.
View Ans
(b);;;;;;Write the structural formulae of the following:
(i);;;;;;;;3-methyl-l pentene
(ii);;;;;;2-methyl-2-pentene
(iii);;;;2,2-dimethyl pentane
(iv);;;;;4-methyl pent-2-yne
View Ans
(C) Identify a simple chemical test that can be used to distinguish between the following compounds: (i) I-butyne and 2-butyne (ii) Butane and butene.
View Ans
13.;;(a) (i) Outline the stages in the formation of chloromethane from methane and chlorine at 450;0C .
(ii) Give reason why the chloromethane obtained in (a)(i) is not pure.
View Ans
(b);;;;;;Bromoalkanes may react with alcohol potassium hydroxide solution to form alkenes. Basing on this statement answer the following questions:
(i);;;;;;;;What type of the organic reaction is this reaction?
(ii);;;;;;Write an equation for the reaction of I-bromobutane with alcohol potassium hydroxide. Show all mechanisms involved.
(iii);;;;Draw the structural formula of the alkene obtained by reaction between 2-bromobutane and alcoholic potassium hydroxide.
View Ans
14.;;(a) Briefly explain the following: (i) C-C bonds are all real and intermediate in length between a single and a double bond in benzene
;(ii) Dry ether is necessary in the preparation and use of the Grignard reagent.
View Ans
(b);;;;;;The chlorination of methyl benzene and 1, I-dimethylethyl benzene yield the following isomers:
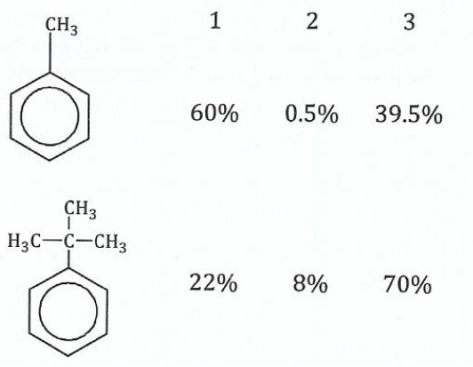
Study the isomers and explain the observed different product ratio.
;
View Ans
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256