THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
132/1 CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 Hours Year: 2014
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.
2. Answer all questions in section A and two (2) questions from section B.
3. Each question carries ten (10) marks in section A and fifteen (15) marks in section B.
4. Mathematical tables and non-programmable calculators may be used.
5. Cellular phones and any unauthorised materials are not allowed in the examination room.
6. Write your Examination Number on every page of your answer booklet(s).
7. For calculations, you may use the following
Rydberg constant, RH = 1.09678x 107m-1
Gas constant, R = 8.31JmoI- 1 K -I or 0.0082 atm mol-1 K -I dm3
GMV at s.t.p. 22.4dm3
1 litre = 1 dm3 =1000cm3
At STP: Temperature = 273K, Pressure = 760mmHg
Planck constant, h=6.63x 10 -34 Js
Velocity of light, C = 3.0 x 108 m/s
Atomic masses H = 1, C = 12, N = 14, O-16, Na-23![]()
1. Nitrogen and oxygen combine endothermic ally at elevated temperature according to the equation 2N 2(g) +O2(g) ![]() 2NO2(g)• If the equilibrium constant for the reaction is 4.3x10-3 at 30000C and I atm, calculate the composition of each in the equilibrium if 2 moles of each nitrogen and oxygen were heated.
2NO2(g)• If the equilibrium constant for the reaction is 4.3x10-3 at 30000C and I atm, calculate the composition of each in the equilibrium if 2 moles of each nitrogen and oxygen were heated.
(b) When 20.85g of PC15 was heated in a sealed tube of 4 dm3 volume, the pressure in the vessel was found to be 1.5 atm. At this pressure it was found that PCI5 dissociated to 80%. Calculate the partial pressure of each gas.
View Ans2. (a) State the following laws:
(i) Graham's law of gas diffusion (ii) Charles' law (iii) Boyle's law.
View Ans(b) Identify two laws in 2(a) above and show how they can be combined to give a single gas equation.
View Ans(c) A chloride of phosphorus is found to diffuse in the gaseous state more slowly by a factor of 2.216 than nitrogen under the same conditions.
(i) Calculate the relative molecular mass of chloride.
(ii) Given that chloride molecule contains one atom of phosphorus, write down its formula.
View Ans3. Define colligative properties and give four examples of those properties.
View Ans(b) Nicotine which is extracted from tobacco leaves is completely immiscible with water at temperature below 60 0 C.
(i) What is the molality of nicotine in an aqueous solution that starts to freeze at-O.45 0 C given that Kf = 1.860cm -l ?
(ii) If this solution is obtained by dissolving 1.92 lg of nicotine in 48.92g of water, what must be the molar mass of nicotine?
(iii) Combustion analysis shows that, nicotine consists of 74.03% C; 8.7% H and
17.27% N by mass. What is the molecular formula of nicotine?
View Ans(a) (i) Write the atomic number of an atom with electronic configuration of 1s 2 2s 2 2p 6 3s 2 3p5 .
(ii) X occurs naturally as 37X and 35k Given that the relative atomic mass of X is 35.5, determines the percentage of 35X and 37X in the sample of element X.
View Ans(b) "The motion of the electron in an atom is not a simple rotation around an orbit, but rather a three dimensional standing wave which obeys Schrödinger equation".
(i) In which atomic model is this statement based?
(ii) Name other two atomic models that attempt to explain the structure of the atom.
© (i) Describe the dual nature of electromagnetic radiation and wave particle duality.
(ii) Calculate the velocity of an electron whose wavelength is 10-9 metres.
View Ans5.(a)(i) Arrange the following coloured lights in order of increasing wavelength: green, blue, red, violet and yellow.
(ii) With reference to a prism spectrometer, briefly explain the term frequency of a line.
View Ans(b) (i)With reference to krypton at ground state, how many electrons have the following set of quantum numbers?
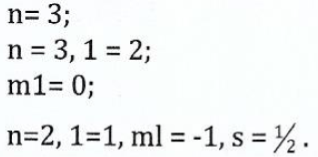
(ii) Briefly explain in molecular orbital terms, the bonding in silane (SiH4).
View Ans6. (a) A solution is prepared from 90g of water and 10.6g of a non- volatile, non-dissociating solute. The vapour pressure of the solution at 60 0 C is found to be 18.91x103Nm-2 , Calculate the approximate molecular mass of the solute given that, the vapour pressure of water at 60 0 C is 19.92Nm-2![]()
(b) Ethanol and water form an azeotropic mixture which boils at 78.1 0 C with 95.6% ethanol. The boiling points of pure ethanol and water are 78.4 0 C and 100 0 C respectively.
(i) Draw a temperature-mole fraction phase diagram of ethanol-water solution. (ii) What happens when a solution of less than 50% ethanol is boiled?
View AnsSECTION B
7. (a) Calculate the standard heat of formation of the reaction C graphite + 2H2(g) →CH4(g) from the following sets of data:
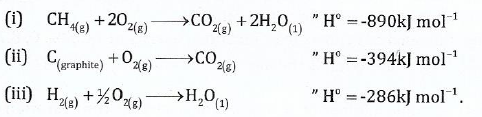
(b) NaCl(s) can be formed directly from sodium and chlorine elements via the reaction:
Na(s) + 1/2Cl 2(g)—»NaCl(g ) " H Of = -411kJmoI -1 .
Construct a well labelled Born-Haber cycle for the formation of NaCl
View Ans8. (a) For the equilibrium: 2NO + O2(g) ![]() 2NO2(g) AHO=-115kJ
2NO2(g) AHO=-115kJ
left—right established in a closed vessel at a fixed temperature, the equilibrium constant reaction has a value of 15mol-1
(i) Write an expression for the equilibrium constant K.
(ii) What does the magnitude of K indicate?
(iii) What will be the effect on the value of the equilibrium constant K if the temperature is increased? Give reason for your answer.
(iv) Calculate the equilibrium concentration of N02 when the equilibrium concentrations of NO and 02 are both 0.1mol-1.
View Ans(b) The value for equilibrium constant, K, for the following reaction equation is equal to 1. acid +alcohol ![]() ester +water.
ester +water.
(i) Predict the maximum yield of ester under the given value of K, and give reason why this yield might not be achieved in practice.
(ii) Will the addition of a catalyst increase the yield of ester? Give reason.
(iii) Comment on the statement that, "increasing the concentration of the alcohol ![]() in the reaction mixture would increase the yield of ester by altering the value of K".
in the reaction mixture would increase the yield of ester by altering the value of K".
9.(a) State four postulates of the kinetic theory of gases.
View Ans(b) Define root mean square speed of gas molecules.
View Ans(c) The root mean square speed of hydrogen molecules at a fixed temperature is ![]() 1600m/s. What is the root mean square speed of oxygen molecules at the same temperature?
1600m/s. What is the root mean square speed of oxygen molecules at the same temperature?
10. (a) Giving reason; explain for each of the following observations:
(i) The boiling points of water, ethanol and ethoxyethane are in the reverse order of their relative molecular masses unlike those of their analogous sulphur compounds; H2S, C2H5SH and C2H5SC2H5.
(ii) BF3 is non-polar but NF3 is polar
(iii) Aluminium fluoride has much higher melting point than aluminium chloride.
View Ans(b) Given that X, Y and Z represent elements of atomic numbers 9, 19 and 34, (i) Write the electronic configuration of X, Y and Z.
(ii) Predict the type of bonding which you would expect between X and Y; Y and z.
(iii) Predict giving reasons for relative volatility, electrical conductance and solubility in water of the compounds formed between X and Y compared to that formed between X and Z.
View AnsSECTION C
11. (a) Briefly explain the following:
(i) Chain reaction.
(ii) Chain initiating step.
(iii) Chain propagation step
(iv) Chain terminating step.
View Ans(b) (i) Why does benzene molecule show extra stability?
(ii) What is resonance and how is it applicable in benzene?
View Ans(c) Suggest suitable chemical tests to distinguish between the following:
(i) Hexane and 2-hexene.
(ii) Propyne and propene.
(iii) I-pentyne and 2-pentyne.
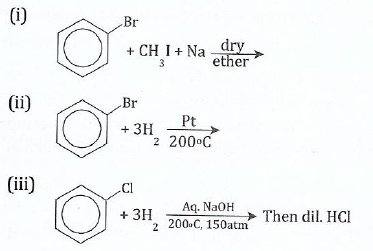
View Ans12 (a) Give the organic product in each of the following:
(b) Show the mechanism for the nitration of benzene.
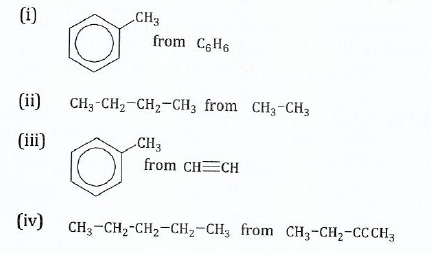
View Ans(c) With the help of chemical equations show how you can prepare the following:
13. Briefly explain why (CH3)3CBr reacts by SNI mechanism while CH3CH2Br reacts by N2 mechanism.
View Ans(b) The ease for the nucleophilic substitution reaction of alkyl halide R-X with OH is the order C-I>C-Br>C-CI>C-F. Explain this trend.
View Ans(c) Give all possible isomers of the compound C5H10Br2 and their corresponding IUPAC names.
View Ans14. What is ozonolysis?
View Ans(b) ![]() A hydrocarbon having a molar mass of 96gmol-1 and molecular formula C7H12 was ozonolysed and then hydrolysed in the presence of zinc. The products of ozonolysis were ethanol, propanone and glyoxal.
A hydrocarbon having a molar mass of 96gmol-1 and molecular formula C7H12 was ozonolysed and then hydrolysed in the presence of zinc. The products of ozonolysis were ethanol, propanone and glyoxal.
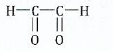
Determine the structure of the hydrocarbon and show ozonolysis of the compound.
View Ans(c) Two isomeric hydrocarbons P and Q have molecular formula C9H12. On oxidation, P gives monocarboxylic acid, when treated with soda lime yields benzene. Q is oxidized to give tricarboxylic acid and can undergo nitration reaction to give two mono-nitro derivatives.
(i) Write down the structural formula of P and Q
(ii) Write an equation to show how Q is oxidized to give tricarboxylic acid.
(iii) Name the compound which is formed when P undergoes oxidation.
View Ans
(b) A vertical spring fixed at one end has a mass of 0.2kg and is attached at the other end.
Determine the:
(i) Extension of the spring. (ii) Energy stored in the spring.
View Ans(c) The displacement of a particle from the equilibrium position moving with simple harmonic motion is given by x = 0.05 sin6t where t is time in seconds measured from an instant when x = 0. Calculate the:
(i) Amplitude of oscillations.
(ii) Period of oscillations.
(iii) maximum acceleration of the particle.
View Ans5. (a)(i) Define the universal gravitational constant.
(ii) How is gravitational potential related to gravitational field strength?
View Ans(b) (i) Write down an expression for the acceleration due to gravity (g) of a body of mass m which is at a distance r from the centre of the earth.
(ii) If the earth were made of lead of relative density of 11.3, what would be the value of acceleration due to gravity on the surface of the earth?
View Ans(c) (i) Why does the value of acceleration due to gravity (g) change due to the change in latitude? Give two reasons.
(ii) A rocket is fired from the earth towards the sun. At what point on its path is the gravitational force on the rocket zero?
View Ans6. (a) Define torque and give its S.I unit.
(ii) A disc of moment of inertia 2.5 x 10-4 kgm2 is rotating freely about an axis through its centre at 20rev/min. If some wax of mass 0.04kg is dropped gently onto the disc 0.05m from its axis, what will be the new revolution per minute of the disc?
View Ans(b) Explain briefly why a:
(i) high diver can turn more somersaults before striking the water?
(ii) dancer on skates can spin faster by folding her arms?
View Ans(c) A heavy flywheel of moment of inertia 0.4kgm2 is mounted on horizontal axle of radius 0.01m.If a force of 60N Is applied tangentially to the axle;
(i) Calculate the angular velocity of the flywheel after 5 seconds from rest.
(ii) List down two assumptions taken to arrive at your answer in 6(c) (i).
View AnsSECTION B
7. (a) (i) Give two ways in which the internal energy of the system can be changed.
(ii) List down two simple applications of the First law of thermodynamics in our daily ![]() life.
life.
(b) One mole of a gas expands from volume VI to a volume V2 If the gas obeys the Van der-Waal's equation;
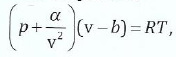
derive the formula for work done in this process.
View AnsANSWER
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania





