THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
132/1 CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 Hours Year: 2013
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.;
2. Answer all questions in section A and two (2) questions from section B.
;3. Each question carries ten (10) marks in section A and fifteen (15) marks in section B.
;4. Mathematical tables and non-programmable calculators may be used.;
5. Cellular phones and any unauthorised materials are not allowed in the examination room.;
6. Write your Examination Number on every page of your answer booklet(s).
7.;;For calculations, you may use the following
Rydberg constant, RH;=;1.09678x 107m-1
Gas constant, R = 8.31JmoI-;1;K;-I;or 0.0082 atm mol-1;K;-I;dm3
GMV;;at s.t.p.;;;;22.4dm3
1 litre = 1 dm3;=1000cm3
At STP: Temperature = 273K, Pressure = 760mmHg
Planck constant, h=6.63x 10;-34;Js
Velocity of light, C = 3.0 x 108;m/s
Atomic masses H = 1, C = 12, N = 14, O-16, Na-23
;
;
1.;;Differentiate nuclear reaction from chemical reaction.;
View Ans
(b);;Complete the following nuclear equation:
;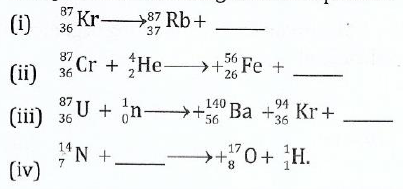
 View Ans
View Ans
(c) The following figure shows the mass spectrum of lead. The highest peaks and the mass numbers of the isotopes are shown. Calculate the average atomic mass of lead.
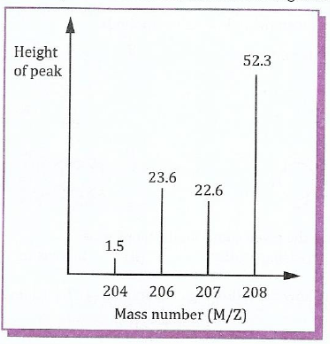
;
View Ans
2.;;(a) Lyman discovered a series of spectral lines for hydrogen in the ultra-violet region of the electromagnetic spectrum. What value must n1 have for this series? Give a reason for your answer.
View Ans
(b);;;;;;Calculate the energy of a line in the Lyman series with ni = land n2 = 00.
View Ans
(c) An experimental iodine laser emits light of wavelength 1.315gm. Calculate the frequency of this light and the energy per photon.
View Ans
3.;;Predict the hybridization of the following:
(i);;;;;;PCl5
(ii);;;SF6
(iii);CO2
(iv);BC13
View Ans
(b);;;;;;Arrange the following substances in order of increasing melting points:;
C02, H20, CO, H2;and Ar.
View Ans
(c) Use VSEPR theory to predict the molecular geometry of the species PCL, NH3;and PC15.
View Ans
4.;;State two characteristics of compounds which are suitable for steam distillation.
View Ans
(b);;;;;;An aromatic compound Z was steam distilled at 98.60;C and 1 atmospheric pressure. The distillate was found to contain 25.5 grams of water and 7.4 grams of aromatic compound Z. Given that the saturated vapour pressure of water at 98.60C is 720mmHg, calculate the relative molecular mass of the aromatic compound.
View Ans
(c);;;;;;Benzene (C6H6) and toluene form a nearly ideal solution. At 313K the vapour pressure of pure benzene is 150mmHg and of pure toluene is 50mmHg. Calculate the vapour pressure of a mixture of these two liquids containing equal masses at the given temperature.
View Ans
5.;;Define the following:
(i);;;;;;;Molar volume of a gas at s.t.p
(ii);;;;;;Dalton's law of partial pressure.
View Ans
(b);;;;;;Two has burettes, one containing 10cm3;of sulphur dioxide (S02) and other containing 30cm3;of hydrogen sulphide (H2S) both at I atmosphere and at OOC are initially separated by a stop cork. The stop cork is then opened and the two gases are allowed to mix according to the reaction SO2(g);+ 2H2S(S)→3S(s);+ 2H20(l)
Calculate the final pressure (in atmospheres) after the reaction has ended and the apparatus has regained its temperature of OOC. (Assume liquid water does not exert pressure).
View Ans
6.;;Distinguish between the following:
(i);;;;;;Reaction quotient and equilibrium constant.
(ii);;;;Chemical equilibrium and physical equilibrium.
View Ans
;(b) (i) The Kc value for the reaction 2NO(g);+ Cl2;; ;2NOCl(g);is
;2NOCl(g);is
4.6x 104;dm3;moElat 298 Kelvin. Calculate the KP value for this equilibrium.
(ii) Calculate the KP value for the equilibrium 2NOC12(g); 2NO +Cl2(g);;at 298 Kelvin.
2NO +Cl2(g);;at 298 Kelvin.
View Ans
(c);;(i) For equilibrium reaction N2(g) +3H2(g); ;2NH3(g);derive KP and Kc relationship if the pressure of the mixture is PT;and volume is VT.
;2NH3(g);derive KP and Kc relationship if the pressure of the mixture is PT;and volume is VT.
View Ans
SECTION B
7.;;;(a) Three elements; F, G, and H have atomic numbers 17, 18 and 19 respectively.
(i);;;;Write electronic configuration of each element.
(ii);;;;;What type of ion each element is expected to form?
(iii);;;;In which group and period would each element be placed?
View Ans
(b);;;;;Study the following hypothetical elements in a periodic table and then answer questions that follow.
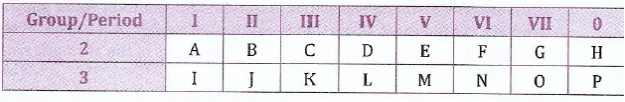
Using letters indicated in the table, identify the following:
(i);;;;;The element which is the most electronegative.
(ii);;;;;A pair of elements that is likely to form the strongest electrovalent bond.
(iii);;;Two elements which are likely to have strongest reducing properties.;
(iv);;;Two elements which form neither negative nor positive ions.
View Ans
8.;;(a) With reference to Modern periodic table, briefly explain the following
(i);;;;;Diagonal relationship.
(ii);;;;Anomalous behaviour
View Ans
(b);;;;;Explain how the hydrides of period 3 elements react with water.
View Ans
(c);;;;;;The following are;some oxides of elements Of;;period;;;;3:
Nap,;;A1203, P205, Si02;and MgO. Arrange them in the order of
(i);;;;;;;Increasing in basic characters
(ii);;;;;Decreasing in ionic characters
View Ans
9.;;(a) (i) What is the difference between the first and the second ionization energies of electron?
(ii) The first and the second ionization energies for the sodium atom are 493kJ and 4560kJ respectively. Account for such a big difference between the two ionization energies.
View Ans
(b);;;;;By using chemical reactions, show how sodium reacts with the following:
(i);;;;;;;ethanol (ii) ammonia (iii) oxygen;;;;;;;;;;(vi) water
View Ans
10.;;;;;;(a) State the law of mass action.
View Ans
(b);;;;;One mole of PCI5 was introduced into a closed vessel ata certain temperature. By the time equilibrium was established, it was found that PC15 was 10% dissociated and the total pressure in the vessel was 4 atmospheres. Calculate:
(i);;;;;;;KP value at the temperature of the experiment
(ii);;;;;The total pressure at which PC15 was 20% dissociated.
View Ans
11. (a) Complete the following equations for reactions of the alkanes:
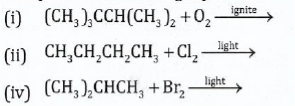
View Ans
;(b);;;;;;Write any three structural isomers of alkylicyclohexane of molecular formula C6H12.
View Ans
(c);;;Write equations for the preparation of alkenes from the following starting materials, indicate the conditions required and if there is more than one product, indicate which one is major.

View Ans
12. Write the Structure of the functional groups of the following sets of compounds:
Write the Structure of the functional groups of the following sets of compounds:
- Alkanes;;;;;;;;;;;;;;;;;;;;;;;;;;;; (ii) alkenes
;;;;;(iii) alkynes;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;(iv) alcohols
;;;;;(v) ketones;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;(vi) aldehydes
;;;;(vii) carboxylic acid;;;;;;;;;;;;;;;;;(viii)tertiary amines
View Ans
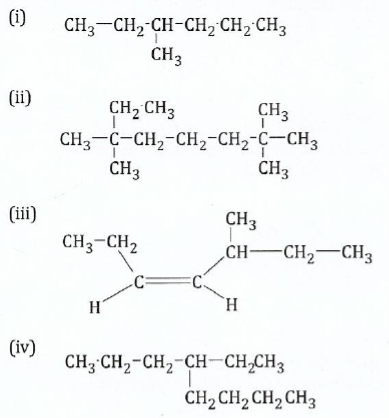
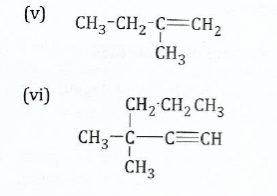
(b) Give the IUPAC name of the following compounds
View Ans
13.;;;;;With support of chemical reactions show how the following compounds can be prepared from ethanol as source of carbon atoms:;
(a);;;;;;;;Benzene
View Ans
(b);;;;;;;Ethyl benzene
View Ans
14.;;;;;(a) Write the structure of the major products for the reaction of gaseous hydrogen bromide with the following:
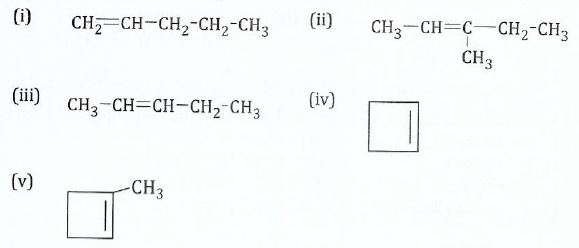
View Ans
;(b);;;;;;;Explain how you can distinguish the following:
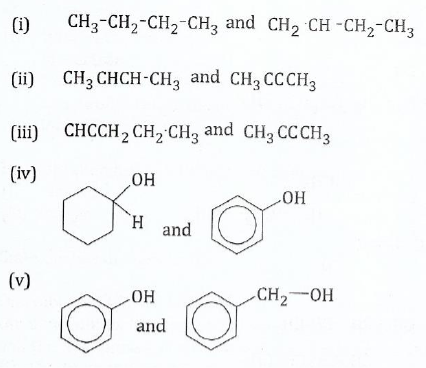
;
View Ans
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256