THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
132/1 CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 Hours Year: 2011
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.
2. Answer all questions in section A and two (2) questions from section B.
3. Each question carries ten (10) marks in section A and fifteen (15) marks in section B.
4. Mathematical tables and non-programmable calculators may be used.
5. Cellular phones and any unauthorised materials are not allowed in the examination room.
6. Write your Examination Number on every page of your answer booklet(s).
7.For calculations, you may use the following
Rydberg constant, RH=1.09678x 107m-1
Gas constant, R = 8.31JmoI-1K-Ior 0.0082 atm mol-1K-Idm3
GMVat s.t.p.22.4dm3
1 litre = 1 dm3=1000cm3
At STP: Temperature = 273K, Pressure = 760mmHg
Planck constant, h=6.63x 10-34Js
Velocity of light, C = 3.0 x 108m/s
Atomic masses H = 1, C = 12, N = 14, O-16, Na-23
1.Define the following terms:
(i)Alpha particles
(ii)Beta particles
(ii)Isotope
(iv)Nuclear Fission
View Ans
(b)A radioactive isotope of polonium 2%Po decays according to the following scheme:
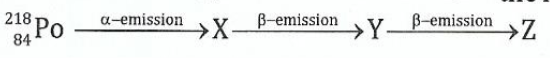
(i)Deduce the mass numbers and atomic numbers of X, Y and Z.
(ii)What is the relationship between the element PO and Z?
View Ans
(c) The element europium, Eu exists in nature as two isotopes; 151Eu with a mass of 150.9196 a.m.u and153Eu with a mass of 152.9096 a.m.u. If the average atomic mass of europium is 151.96 a.m.u, calculate the percentage relative abundances of the two isotopes.
View Ans
2.What is the meaning of the following quantum numbers?
(i) m (ii) s View Ans
(b)List all possible values oft and m when n is 3.
View Ans
(c)When an electron jumps from a certain higher energy level E , to its ground state El, green light in the Balmer series is emitted. If the energy Released during this transition is 4.071 x 10 19 J determine the:
transition is 4.071 x 10 19 J determine the:
(i)Wavelength of the green light
(ii)Higher energy level, E2 from which the electron jumps to the ground energy level El.
View Ans
3.What do you understand by the following types of bonds?
(i)Dative covalent bond.
(ii)Inter-molecular hydrogen bond.
(iii)Covalent bond.
(iv)Intra- molecular hydrogen bond.
View Ans
(b) (i)At 1100C and 454 mmHg. 0.1 lg of ethanoic acid vapour occupies 63.7cm3. At 1560C and 458mmHg, 0.081g of ethanoic acid vapour occupies 66.4cm3. Calculate the molar mass of ethanoic acid in the vapour phase at each temperature.
(ii)Give the interpretation of the results in 3(b)(i)above.
View Ans
4.(a) State the following:
(i)Graham's law ofgas diffusion.
(ii)Dalton's law of partial pressures.
View Ans
(b)A 3.20m3vessel contains a mixture of 86.2g of oxygen and 1.5g of hydrogen at 880C. Calculate the total pressure in the vessel.
View Ans
(c)Gas A of a certain volume diffuses for 580.8s while the same volume of gas J diffuses for 300s under identical experimental conditions. Calculate the relative molecular mass of J ifthe relative molecular mass gas A is 120.
View Ans
5.(a) (i) State the partition law.
(ii) Explain the meaning of miscible solutions.
View Ans
(b)18g of compound X distribute themselves between water and equal volume of an immiscible solvent Y so that 2g of X are in water. Calculate to the nearest integer, the percentage of X left in water if 1000cm3of water containing lg of X are extracted by one litre of Y.
View Ans
6.(a) State the following:
(i) Equilibrium law
(ii) Le Chaterlier's law
View Ans
(b) In the preparation of ethylethanoate shown in the equation below, concentrated H2S04is often added to the mixture.
In the preparation of ethylethanoate shown in the equation below, concentrated H2S04is often added to the mixture.

(i)State two (2) functions of concentrated H2S04in the production of the compound.
(ii)What will be the effect of adding NaOH(aq) instead of conc. H2S04 in the production of compound?
View Ans
(c) (i) When 1.00moI/dm3of CH3COOH were heated with 0.18 mol. of C2H50H in a Idm3closed vessel, 0.829mol. CH COOH remained at equilibrium. Calculate the value of K .
(ii) What mass of ethylethanoate should be present in the equilibrium mixture formed under the same experimental conditions as 6(b)(i) above if 0.30 moles of ethanol were heated with 0.20 moles of ethanoic acid in 1.0dm3closed vessel?
View Ans
7. (a) Explain why the first element in each group of the main periodic table shows properties which are not exhibited by other group elements.
View Ans
(b)Account for the following observations and give chemical reactions whenever
necessary:
(i)The second electron affinity is always positive
(ii)Magnesium carbonate decomposes readily when heated while sodium carbonate has no action to heat.
(iii)Alluminium utensils rust quite easily.
(iv)Water is covalent compound but has a high boiling point.
(v)Hydrogen gas is evolved when magnesium metal is placed in a beaker containing ammonium chloride.
View Ans
(c)Justify the periodicity of properties of elements in the periodic table based on electronic configuration.
(ii)What are s, p and d block elements of periodic table?
View Ans
8.(a)Account for the following:
(i) Zinc and iron are both d-block elements but iron can be magnetized while zinc cannot.
(ii)Iron (Il) sulphate is green while zinc sulphate is white.
View Ans
(b)(i) Manganese is said to exhibit variable oxidation numbers. List them.
(ii) Write the electronic configuration of manganese.(Atomic number = 25)
(iii)Give examples of compounds in which manganese exhibits different oxidation state. (Give example in each case.)
View Ans
9. (a) State the following:
(i) Zeeman effect
(ii) Pauli's exclusion principle
(iii) Hund's rule of maximum multiplicity.
View Ans
(b) (i) What is effective nuclear charge?
(ii) The first ionization energy of aluminum is less than that of magnesium. Giving reasons compare the second ionization energy of aluminum to that of magnesium.
View Ans
(c) Explain why the CI-C-O bond angle in C12CO is 1240C and not 1200.
View Ans
10.(a)Account for the uniqueness of hydrogen in the periodic table.
View Ans
(b)(i) List all the isotopes of hydrogen and explain how they differ from one another.
(ii)Give the products of the following reactions:
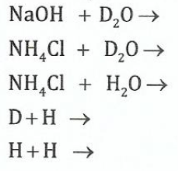
View Ans
© Distinguish between ortho and para hydrogen.
View Ans
11.(a) Name the following compounds according to the IUPAC system:
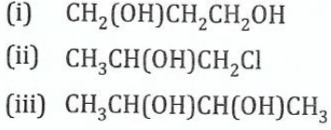
View Ans
(b)Compound X containing a carbon-carbon double bond would react with the following reagents:
(i) Y to form 2 - bromo - 2 - methylbutane
(ii)Z to form (CH3)2CHC(OH)(CH3)2
(iii)W to form 2 - chloro -2 - methylbutane.
Name compound X and reagents Y, Z and W
View Ans
(c)Indicate a chemical test which may be used to distinguish the members of each of the following pairs. Indicate of the pair that gives the positive or greater reaction.
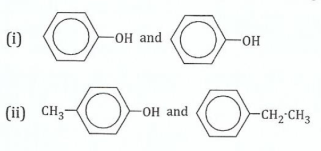
View Ans
12. (a) Explain briefly the following terms:
(i)Nucleophiles
(ii)Nucleophilic substitution
View Ans
(b)Arrange the following compounds in order of increasing easiness in substitution of the halogen atom by an electrophile. Give reasons for your arrangement
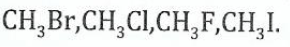
View Ans
(c)Explain the fact that nucleophilic substitution reaction in I-bromobutane is bimolecular whereas nuncleophilic substitution reaction in 2-methyl-2bromopropane is monomolecular.
View Ans
13.(a) Write the structural formulae of the following compounds:
(i) 2,4-dinitrophenoI (ii) p-aminophenol.
(b)Give the names and structures of dimethyl benzenes.
View Ans
(c)Explain with the help of chemical equations which dimethyl benzene in 131b) above will yield:
(i)one (l)mononitrito product
(ii)two(2) mononitrito product
View Ans
14.Give the organic product(s) of each of the following reactions:
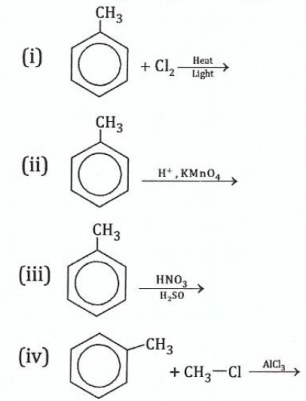
View Ans
(b)Describe the chemical test you would carry out to distinguish the following compounds:
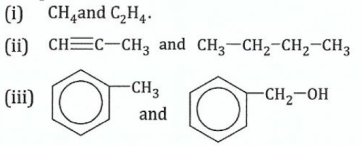
View Ans
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256