THE UNITED REPUBLIC OF TANZANIA MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
FORM TWO SECONDARY EDUCATION EXAMINATION, 2009
0032 CHEMISTRY
TIME: 2 HOURS
INSTRUCTIONS
1. This paper consists of three sections A, B and C.
2. Answer all questions in spaces provided for each question.
3. Write your examination number on the top right hand corner of every page.
4. All writing must be done in “black or blue pen” except for the diagrams which must be in pencil.
5. Cellphones and calculators are not allowed in the examination room.
6. The following constants may be used:
Atomic masses: H = 1, C = 12, O = 16, and Na = 23.
SECTION A
1. Write down the letter of the correct answer against each question.
(i)The fixed volume (20 cm3) of distilled water in the laboratory can be measured by using:
- Beaker
- Burette
- Pipette
- Small measuring cylinder
Choose Answer :
(ii) Domestic utensils made of iron undergo rusting when exposed to:
- Air and fire
- Air and oil
- Air and water
- Air and soil
Choose Answer :
(iii) The source of energy which when used can be made to be put into use again is known as:
- Fuel
- Non-renewable energy
- Renewable energy
- Solar energy
Choose Answer :
(iv) A student who gets burnt accidentally in the chemistry laboratory would be given one of the following as first aid:
- Antibiotic solution
- Nitric acid
- Petroleum jelly
- Potassium permanganate
Choose Answer :
(v) The chloride ion (Cl-) differs from chlorine atom because chloride ion has:
- Less electrons
- Less protons
- More electrons
- More protons
Choose Answer :
(vi) The percentage by mass of nitrogen in (NH4)2CO3 is:
- 28.0
- 29.1
- 37.5
- 96.0
Choose Answer :
(vii) The elements which are found in group VIII of the periodic table are known as:
- Metals
- Noble gases
- Non-metals
- Right elements
Choose Answer :
(viii) Isotopes are atoms which have:
- Different mass number
- Different number of electrons
- Different number of protons
- The same number of neutrons
Choose Answer :
(ix) The process used to separate a mixture of salt and water is:
- Evaporation
- Filtration
- Simple distillation
- Sublimation
Choose Answer :
(x) Which of the following chemical species have the same number of electrons?
- Cl, Be, He and O2-
- K+ , Ca2+ , Cl- and Ar
- Na+ , Mg2+ , C13 and Ar
- O2- , F- , S2- and Cl-
Choose Answer :
SECTION B
2. You are provided with two lists, A and B. Choose a word(s) from list B which matches the statement or phrase in list A.
| List A | List B |
| (i) . . . . . . . Covalent bond (ii) . . . . . . . Combining capacity of an element (iii) . . . . . . . Destructive distillation of wood (iv) . . . . . . Increases from left to right across the period in the periodic table (v) . . . . . . Liquid metal (vi) . . . . . "Pop sound" (vii) . . . . . . Re-lights a glowing splint (viii) . . . . . Turns lime water milky (ix) . . . . . The product of heating iron and sulphur (x) . . . . . . The study of composition of matter and its behaviour | - Carbon dioxide
- Carbon monoxide
- Charcoal
- Chalk
- Chemical change
- Chemical composition
- Chemistry
- Electron sharing
- Electron transfer
- Electro-negativity
- Hydride
- Mercury
- Oxygen
- Valency
|
View Ans
3. (a) For each of the following classes of fire, state the burning material(s)
(i) Class A fire
(ii) Class B fire
(iii) Class C fire
View Ans
(b) Fire can be prevented by (Mention four)
View Ans
4. Use the following information about elements F, G, L, M and J shown in the Table below to answer the questions that follow:
| Element | Atomic number | Atomic mass |
| F | 8 | 16 |
| G | 9 | 19 |
| L | 11 | 23 |
| M | 6 | 12 |
| J | 18 | 40 |
(a) (i) Write down the electrons configuration of elements F, G, L and J.
(ii) How many neutrons are present in element G?
View Ans
(b) Write the chemical symbols for the following elements:
(i) Silver
(ii) Lead
(iii) Tin
View Ans
5. (a) State the meaning of apparatus.
View Ans
(b) Draw and state one function for each of the following laboratory apparatus:
| Apparatus | Diagram | Function |
| Burette |
|
|
| Pipette |
|
|
| Measuring cylinder |
|
|
| Beaker |
|
|
| Test tube |
|
|
View Ans
6. Oxygen is obtained by heating a metal chlorate in the presence of a catalyst.
(a) Write down the name of the:
(i) Metal chlorate used
(ii) The product formed other than oxygen
View Ans
(b) Write the:
(i) Formula and the IUPAC name of the catalyst used.
(ii) Word equation for this reaction.
View Ans
(c) Draw a large and well labelled diagram for this laboratory preparation of oxygen.
View Ans
7. (a) Define the term "chemical formula".
View Ans
(b) Write down the chemical formula of the following compounds:
(i) Sodium carbonate ten water
(ii) Calcium hydroxide
(iii) Sulphuric acid
View Ans
(c) Give the IUPAC names for each of the following compounds:
(i) CU2O
(ii) Na2SO4
(iii) NH4NO3
(iv) Fe2O3
(v) H2O
View Ans
8. (a) What is air?
View Ans
(b) Mention the composition of air with their percentage composition:
View Ans
(c) Give four reasons why air is a mixture.
View Ans
9. Study the following diagram carefully and then answer the questions that follow:
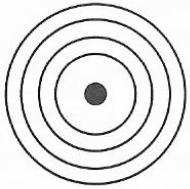
(a) What is the name given to the arrangement of electrons around the nucleus?
View Ans
(b) What is the name of the layers in which the electrons are arranged?
View Ans
(c) If each layer can hold a maximum number of electrons given by formula 2n2, what does "n" represent?
View Ans
(d) By using the formula presented in Cc) above, calculate the number of electrons in:
(i) K - Layer
(ii) L - Layer
(iii) M - Layer
(iv) N - Layer
View Ans
10. (a) Give the method used to separate the following mixtures:
(i) Kerosene and water
(ii) Alcohol (ethanol) and water
(iii)Iron filings and sand
View Ans
(b) Water is a chemical compound. Give four reasons to support this fact.
View Ans
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256