Candidate's Examination Number. . . . . . . . . . . . . . . .
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA
FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Time: 2:30 Hours Year: 2024
Instructions
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all the questions in each section.
- All answers must be written in the spaces provided.
- All writing must be in blue or black ink, except drawings which must be in pencil.
- Communication devices and any unauthorized materials are not allowed in the assessment room.
- Write your Assessment Number at the top right corner of every page.
- Where necessary the following constants may be used: H=1, C=12, O=16
| FOR ASSESSOR'S USE ONLY | ||
| QUESTION NUMBER | SCORE | ASSESSOR'S INITIALS |
| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
| 6. | ||
| 7. | ||
| 8. | ||
| 9. | ||
| 10. | ||
| TOTAL | ||
| CHECKER'S INITIALS | ||
SECTION A (15 Marks)
Answer all questions in this section.
1.For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter in the box provided.
(i) How are the different atoms which occupy the same group and period called?
- Isotopes
- Isomers
- Monomers
- Isobers
(ii) Which one of the following is not a suitable means of separating the components of air?
- Chemical means
- Physical means
- Freezing method
- Precipitation method
(iii) Which source of flame produces a non-luminous flame?
- Candle
- Kerosene stove
- Tin lamp
- Bunsen burner
(iv) How can water be changed from vapour to liquid state?
- By sublimation
- By evaporation
- By melting
- By condensation
(v) Why is water regarded as the universal solvent?
- Because it is found all over the world
- Because it contain hydrogen and oxygen elements
- Because most of substances dissolve in it
- Because it contains a variety of minerals
(vi) What is the total number of electrons in hypothetical ion Q2+ whose atomic number is 12?
- 12
- 14
- 10
- 24
(vii) Which one of the following is not a part of the Bunsen burner?
- Jet
- Barrel
- Gas tap
- Air hole
(viii) Which apparatus serves the function of stirring substances?
- Desiccator
- Glass rod
- Spatula
- Deflagrating spoon
(ix) How can contaminants be removed from water'?
- Through purification
- Through sedimentation
- Through electrolysis
- Through decantation
(x) How many protons are there in a molecule of oxygen gas?
- 8
- 17
- 9
- 16
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Time: 2:30 Hours Year: 2023
Instructions
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all the questions.
- Section A and C carry fifteen (15) marks each and section B carries seventy (70) marks.
- All writing must be in black or blue ink except diagrams which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
- Write your Assessment Number at the top right corner of every page.
- The following atomic masses may be used: H = l , C = 12, O = 16, Cl = 35.5.
| FOR ASSESSOR'S USE ONLY | ||
| QUESTION NUMBER | SCORE | ASSESSOR'S INITIALS |
| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
| 6. | ||
| 7. | ||
| 8. | ||
| 9. | ||
| 10. | ||
| TOTAL | ||
| CHECKER'S INITIALS | ||
SECTION A (15 Marks)
Answer all questions in this section.
For each of the items (i) - (x), choose the correct answer from the given alternatives and write its letter in the box provided.
(i) What are the common activities done in the chemistry laboratory?
- Exhibitions
- Demonstrations
- Exercises
- Experiments
(ii) The following substances are constituents of a First Aid Kit in the chemistry laboratory, except:
- petroleum jelly
- iodine tincture
- cotton wool
- plaster of paris
(iii) What is the suitable method for separating a mixture of sand and ammonium chloride?
- Magnetization
- Decantation
- Sublimation
- Simple distillation
(iv) Which one is an example of liquid solutions?
- Dental amalgam
- Fresh milk
- Alloys
- Vinegar
(v) Why do the ships often have blocks of magnesium attached to their hull?
- To improve appearance of the hull.
- To make the hull stronger.
- To give sacrificial protection to the hull.
- To weigh down the ship in the water.
(vi) Given a task of preparing hydrogen gas in the laboratory, which complete set of apparatuses will you use?
- Thistle funnel, flat-bottomed flask, pipette, water trough, beehive stand and a gas jar.
- Thistle funnel, flat-bottomed flask, delivery tube, water trough, beehive stand and burette.
- Thistle funnel, flat-bottomed flask, delivery tube, water trough, beehive stand and a gas jar.
- Thistle funnel, flat-bottomed flask, delivery tube, measuring cylinder, beehive stand and a gas jar.
(vii) What is the role of charcoal in filter elements?
- To kill germs
- To sediment impurities
- To coagulate impurities
- To trap dust particles
(viii) Why is wind considered a promising source of energy for the future?
- It does not produce harmful gases.
- It is easily stored.
- It is harnessed without chemical reaction.
- It is a renewable source of energy.
(ix) Given that, the amount of heat gained by water after a complete combustion of 46 g of ethanol (C2H50H) is 8.4 kJ, what is the energy value of ethanol in J/ ?
- 182.0
- 182.7
- 182.6
- 182.8
(x) The following sets of radicals have oxidation states of either -l or -2 except;
- hydroxide, carbonate, nitrate, phosphate, chlorate and sulphite.
- hydroxide, sulphate, carbonate, nitrate, nitrite, chlorate and sulphite.
- hydroxide, carbonate, nitrate, chlorate and hydrogen carbonate.
- hydroxide, carbonate, nitrite, chlorate, sulphate and nitrate.
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA
FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in the spaces provided
- All writing must be in black or blue ink except diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used: H= 1, N= 14, O= 16, S=32, Ca=40.
1 For each of the items (i) – (x), choose the correct answer from the given alternatives and write its letter in the box provided.
(i)Identify the set of chemistry products which are used for domestic cleanliness
- Tooth paste, oils, detergents and deodorants
- Soap, deodorants, tooth paste and fuel
- Detergents, soap, tooth paste and deodorants
- Drugs, tooth paste, soap and oils
(ii) During practical work a measuring cylinder was used to prepare oxygen by decomposing hydrogen peroxide. What is the function of the cylinder in this experiment?
- To measure volume
- To measure weight
- To measure width
- To measure volume length
(iii) Your friends were arguing about the scientific procedure that follows after data interpretation. Which stage will you suggest to your friends?
- Observation
- Hypothesis
- Conclusion
- Experimentation
(iv) The teacher was demonstrating an experiment by dissolving sodium chloride in water until the solute was not dissolving any more. What type of solution formed at the end of the experiment?
- Saturated
- Unsaturated
- Super saturated
- Suspension
(v) A large percent of air is composed of
- Nitrogen
- Noble gases
- Carbon dioxide
- Oxygen
(vi) John and Asha were debating about the process that are involved during simple distillation. What processes will you recommend to them?
- Filtration and decantation
- Condensation and decantation
- Evaporation and filtration
- Evaporation and condensation
(vii) Form Two student discovered that it is impossible to light fire in a vacuum due to absence of a certain gas. What comment can you give to the student?
- Nitrogen is missing
- Oxygen is missing
- Carbon dioxide is missing
- Hydrogen is missing
(viii) Atomic structures of all elements consist of electrons, protons and neutrons except that of
- Hyadrogen
- Nitrogen
- Oxygen
- Carbon
(ix) When referring to the modern Periodic Table, the transition elements are found between:
- Group I and II
- Group I and III
- Group II and III
- Group III and IV
(x) Given that, element “M” with electronic configuration of 2:8:3 combines with element “G” with electronic configuration of 2:6 to form a compound; What is the chemical formula of the compound formed.
- G3M2
- M2G3
- G2M3
- M3G2
Student's Assessment Number .....
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA
FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Time: 2:30 Hours Year: 2021
Instructions
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in the spaces provided.
- Section A carries twenty (20) marks and section B carries eighty (80) marks.
- All writing must be in black or blue ink except diagrams which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
- Write your Assessment Number at the top right corner of every page.
- The following atomic masses may be used: H = 1, 0 = 16, S = 32, Ca = 40, Na = 23.
| FOR ASSESSORS' USE ONLY | ||
| QUESTION NUMBER | SCORE | ASSESSOR'S INITIALS |
| 1 | | |
| 2 | | |
| 3 | | |
| 4 | | |
| 5 | | |
| 6 | | |
| 7 | | |
| 8 | | |
| 9 | | |
| 10 | | |
| TOTAL | | |
| CHECKER'S INITIALS | | |
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter in the box provided.
(i) Which particles contribute the net charge inside the nucleus of an atom?
- Protons
- Neutrons
- Electrons
- Nucleons
(ii) Which of the following is not a man-made product of applying chemistry'?
- Fertilizer
- Milk
- Sugar
- Vaccines
(iii) How is the amount of air entering in the Bunsen burner controlled?
- By adjusting the opening of the barrel.
- By adjusting the opening of the collar.
- By adjusting the opening of the jet.
- By adjusting the opening of the base.
(iv) How do the chemists refer to a mixture of milk and water?
- Emulsion
- Suspension
- Miscible solution
- Immiscible solution
(v) Why is it necessary to boil drinking water?
- To remove oxygen.
- To remove impurities.
- To make it tasteless.
- To kill micro-organisms.
(vi) Which of the following indicates a pair of isotopes?
- 4020X and 4018X
- 3919X and 4020X
- 126X and 126X
- 3517X and 3717X
(vii) Which of the following are the products of the reaction of sodium metal with water?
- Sodium oxide and hydrogen gas.
- Sodium hydroxide and water.
- Sodium oxide and water vapour.
- Sodium hydroxide and hydrogen gas.
(viii) !low does the covalent bond form?
- By combining opposite charged atoms.
- By loss of electrons between ions.
- By sharing of valence electrons.
- By force of attraction of atoms.
(ix) flow can one prevent rusting in fragile instruments like cameras?
- By using silica gel.
- By using ethanol.
- By galvanization.
- By using oil.
(x) What is the maximum number of electrons in the innermost shell of an atom?
- 3
- 8
- 2
- 1
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA
FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Time: 2:30 HoursYear: 2020
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.
2. Answer all questions in the spaces provided.
3. Section A carries twenty (20) marks and section B carries eighty (80) marks.
4. All writing must be in black or blue ink except diagrams which must be in pencil.
5. Cellular phones and any unauthorised materials are not allowed in the assessment room.
6. Write your Assessment Number at the top right corner of every page.
7. The following atomic masses may be used: H = 1, N = 14, 0 = 16, S = 32, Ca = 40.
SECTION A (20 Marks)
Answer an questions in this section.
I .For each of the items (1) (x), choose the correct answer from among the given alternatives and write its letter in the box provided.
(i) The net charge inside the nucleus of an atom is contributed by
- protons
- neutrons
- electrons
- all nucleons.
(ii) Why oxygen as one of the components of air is unique?
- It support combustion.
- It is a diatomic gas.
- It forms the largest part of the air.
- It has the largest density.
(iii) Which material is not involved in respiration?
- Carbon dioxide
- Nitrogen
- Oxygen
- Water
(iv) Which element causes permanent hardness of water when combined with sulphate?
- aluminium
- magnesium
- potassium
- sodium.
(v) Carbon dioxide. Oxygen, Nitrogen and Hydrogen Sulphide are
- major components of air.
- covalent compounds.
- divalent gases.
- ionic compounds.
(vi) Which common feature is associated with elements of the same group?
- Equal number of protons
- Equal number of electrons
- Equal number of valence electrons
- Equal number of shells.
(vii) The oxidation state of metallic elements is always
- negative
- Neutral
- positive
- zero.
(viii) An isotope of Lead with atomic number of 82 and mass number of 207 has
- 82 protons, 125 neutrons and 82 electrons.
- 25 protons, 82 neutrons and 125 electrons.
- 82 protons, 207 neutrons and 125 electrons.
- 207 protons, 207 electrons and 82 neutrons.
(ix) Which conditions are necessary for iron nails to rust?
- oxygen and moisture
- carbon and oxygen
- carbon dioxide and oxygen
- oxygen and nitrogen.
(x) Which decision should be made if the results of an experiment do not support the stated hypothesis?
- To use the results as an idea for further testing.
- To ignore the results and set a new experiment.
- To repeat the experiment in the same way.
- To identify a new problem.
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA
FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Duration: 2:30 Hours
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) What is the best way of keeping a clean test tube after use?
- Keeping it in water
- Keeping it on a test tube holder
- Keeping it in a basin for test tubes
- Keeping it on a test tube rack.
(ii) Which one of the following does not involve the processes of urban water treatment and purification?
- Sedimentation
- Distillation
- Filtration
- Chlorination.
(iii) Why hydrogen gas is not a constituent of air?
- Because of being water soluble
- Because of being denser than air
- Because of being very light
- Because of being highly flammable.
(iv) Which is the suitable alternative heat source to be used in absence of Bunsen Burner?
- Torch and spirit burner
- Torch and kerosene stove
- Kerosene stove and spirit burner
- Firewood and torch.
(v) Which group and period does the element with 11 electrons belong?
- Group I and period 3
- Group II and period 1
- Group I and period 1
- Group II and period 3.
(vi) What happens when substance A reacts with substance B to form a new substance C?
- Substance A and B are said to have formed a solution
- Substance A and B are said to have undergone a physical change.
- Substance A and B are said to have undergone a chemical change.
- Substance A and B are said to have undergone a dissolution.
(vii) Which components make fire triangle?
- Oxygen, fuel and heat
- Oxygen, nitrogen and heat
- Oxygen, fuel and carbon dioxide
- Oxygen, heat and hydrogen.
(viii) Which state is involved when drying wet clothes?
- Liquid to solid
- Solid to gas
- Gas to liquid
- Liquid to gas.
(ix) Which net charge exists in radicals?
- Zero
- Positive or negative
- Neutral
- Positive and negative.
(x) Why Non – luminous flame is the most applicable flame for heating purposes?
- It is very nosy
- It has no soot.
- It is very hot
- It has air holes open
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATION COUNCIL
CHEMISTRY FTNA 2018
(i) Chemistry is a branch of Science which deals with:
- matter in relation to energy.
- matter in relation to decomposition.
- matter composition and its decomposition.
- properties of conservation of matter.
(ii) Which of the following are the states of matter?
- Gas, liquid and mixture
- Gas, liquid and solid
- Element, compound and mixture
- Element, mixture and gas.
(iii)Which of the following are the main components of a fire triangle?
- Air, temperature and fire
- Oxygen, temperature and fuel
- Oxygen, heat and fuel
- Oxygen, temperature and fire
(iv)The process of removing solid contaminants from water is known as:
- water decantation.
- water solidification.
- water purification.
- water sedimentation.
(v)How many zones are there in a non-luminous flame?
- Four zones
- Two zones
- Three zones
- Five zones
(vi)The process of coating iron or steel with zinc is known as:
- zinc painting.
- alloying.
- tin plating.
- galvanization.
(vii)A certain element has atomic number W and mass number Y. The number of neutrons contained in its nucleus is:
- W
- W-Y
- Y-W
- Y+W
(viii)When a small amount of sugar is dissolved in a glass of water the mixture formed is:
- heterogeneous.
- immiscible.
- suspension.
- homogenous.
(ix)Fainting is a sudden loss of:
- confidence.
- weight of the body.
- water in the body.
- consciousness.
(x)Why is fractional distillation of coal done?
- To remove oxygen in the atmosphere.
- To remove volatile matter.
- To add oxygen in the furnace.
- To add volatile matter.
THE UNITED REPUBLIC OF TANZANIA
NATIONAL EXAMINATIONS COUNCIL FORM TWO NATIONAL ASSESSMENT
032 CHEMISTRY
Time: 2:30 Hours Friday, 17th November 2017
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.
2. Answer all questions in the spaces provided.
3. All writing must be in black or blue ink except diagrams which must be in pencil.
4. All communication devices and calculators are not allowed in the examination room.
5. Write your Examination Number at the top right corner of every page.
6. The following atomic masses may be used: H = 1. N = 14.0 = 16. S = 32. Ca = 40.
(i) Which statement gives a clear meaning of Chemistry?
- The study of matter in relation of energy
- The study of nature and properties of matter
- The study of matter and arrangement of particles
- The study of matter and chemical reaction
(ii) The mass number of an atom is determined by:
- protons and neutrons
- protons and electrons
- neutrons and electrons
- protons alone
(iii)Which of the following is a metal?
- Water
- Chlorine
- Sodium
- Nitrogen
(iv)Air entering the Bunsen burner barrel can be controlled by
- metal ring
- air hole
- metal jet
- air ring
(v) How many atoms are there in a water molecule
- Two
- . Three
- Four
- Five
(vi)Which neutral atom has the same number of eletrons as Mg2+?
- Magnesium
- Sodium
- Neon
- Argon
(vii) The appropriate extinguisher used to put off fire caused by cooking oil is:
(vii) The appropriate extinguisher used to put off fire caused by cooking oil is:
- Water extinguisher
- Carbon extinguisher
- Wet chemical extinguisher
- Dry air extinguisher
(viii) A non-luminous flame is obtained if the hole is:
- fully opened
- partially opened
- closed
- half opened
(ix)Which is the least abundant gas in the air?
- Nitrogen
- Oxygen
- Neon
- Carbon dioxide
(x) The process which produces energy in form of heat and light is called:
- decomposition
- combustion
- distillation
- sublimation
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATION COUNCIL
CHEMISRY FTNA 2016
(i) Chemistry is defined as:
- The scientific study of matter, compounds and chemical reactions
- The scientific study of compounds, mixtures and organic substances
- The scientific study of composition, structure and properties of matter
- The study of relation between human being, medicine and pollution.
(ii) A non-luminous flame is the most applicable flame for heating purposes because:
- It is very noisy
- It has no soot
- It is very hot
- It has no colour
(iii) Matter is defined as anything that has:
- Volume and occupies space
- Mass and occupies space
- Mass and occupies density
- Density and space
(iv) Which of the following process is used in preventing rust of an iron?
- Water
- Boiling
- Salting
- Galvanization
(v) Water is a universal solvent because:
- It is available everywhere
- It boils at 1000C
- It dissolves most of the solutes
- It dissolves all crystals in compounds
(vi) Carbon has two main isotopes, 12 C and 14 C with relative abundance of 98.89% and 1.11% respectively. Calculate the relative atomic mass of carbon.
- 13.5
- 12.01
- 6
- 27
(vii) Class F fire can best be extinguished by using
- Carbon dioxide
- Wet chemical
- Water
- Sand
(viii)Which of the following components can be separated by filtration method?
- Sand and water
- Kerosene and water
- Ethanol and water
- NaCl and water
(ix) Which of the following is the colour change when cobalt chloride paper is used to test the presence of water?
- White when dry and pink when wet
- Blue when dry and pink when wet
- Yellow when dry and pink when wet
- Pink when dry and blue when wet
(x) All domestic utensils made of iron undergo rusting when exposed to
- Air and fire
- Air and oil
- Air and water
- Water and oil
THE UNITED REPUBLIC OF TANZANIA
NATIONAL EXAMINATIONS COUNCIL FORM TWO SECONDARY EDUCATION EXAMINATION
032 CHEMISTRY
Time: 2:30 Hours Thursday, 19th November 2015 a.m.
Instructions
This paper consists of sections A, B and C.
2. Answer all questions in the spaces provided.
3. All writing must be in black or blue ink except diagrams which must be in pencil.
4. All communication devices and calculators are not allowed in the examination room.
5. Write your Examination Number at the top right corner of every page.
6. The following atomic masses may be used: H = 1, 0 = 16, C = 12, N = 14, Na = 23, AI = 27. S = 32, K = 39. Ca = 40, Fe = 56, F = 19, Cu = 64.
(i) Which of the following is the most correct statement about hypothesis?
- A fundamental concept or theory
- A possible explanation to the problem.
- An important statement of a research conclusion
- A stage in data interpretation.
(ii) Which of the following is not among the gases composing air?
- Noble gases
- Carbon dioxide
- Nitrogen
- Hydrogen
(iii) When an atom loses or gains electron, it becomes:
- An ion
- An anion
- A cation
- A charged ion
(iv) A change from gaseous state to solid state without passing through a liquid state is called:
- Deposition
- Sublimation
- Condensation
- Solidification
(v) What is the type of a fire associated with electrical equipment?
- Class E
- Class C
- Class F
- Class B
(vi) Which among the following are the two processes involved during distillation?
- Evaporation and sublimation
- Evaporation and crystallization
- Evaporation and condensation
- Evaporation and decantation
(vii) Which of the following set of nuclide notation represents isotopes?
- 18 X 16 X 19 x
- 18 X 18 X X
- 16 x 18 x 1890x
- 16 X 1789x,
(viii) The chemical used to test the presence of water in a substance is:
A. Cobalt Il oxide B. Cobalt 111 oxide
C.Cobalt chloride D. Copper Il chloride
 (ix) When a burning fuel produces blue colour it means there is:
(ix) When a burning fuel produces blue colour it means there is:
A.adequate supply of oxygen with production of soot
B.inadequate supply of oxygen with production of more heat.
 C. inadequate supply of oxygen with production of soot.
C. inadequate supply of oxygen with production of soot.
D.adequate supply of oxygen with production of more heat.
 (x) Which of the following is the best apparatus for measuring accurately a fixed volume of a given solution?
(x) Which of the following is the best apparatus for measuring accurately a fixed volume of a given solution?
A. Measuring cylinder B. Beaker
C. Pipette D. Burette
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATION COUNCIL
CHEMISTRY FTNA 2014
(i) An isotope of carbon has an atomic number 6 and a mass number of 14, this means that it has:
- 6 protons, 8 neutrons, 6 electrons
- 8 protons, 6 neutrons, 8 neutrons
- 6 protons, 14 neutrons, 6 electrons
- 14 protons, 6 neutrons, 14 electrons.
(ii) Which of the following gives the correct meaning of air?
- Mixture of Nitrogen, Oxygen and dust particles.
- Mixture of Nitrogen, Oxygen and Carbon dioxide.
- C. Mixture of Nitrogen, Oxygen and Water vapour
- Homogenous mixture of gases.
(iii) Why is water a universal solvent?
- It is neither acidic nor basic than any other known liquid.
- It dissolves more substances than any other known liquid.
- It occurs naturally in all the three states of matter than any other liquid.
- It dissolves both organic and inorganic solutes than any other liquid.
(iv) How many numbers of shells are there in Magnesium atom?
- 1
- 2
- 3
- 4
(v) Technicians prefer to use blue flame in welding because:
- It is bright and non-sooty
- It is light and non-sooty
- It is very hot and large
- It is very hot and non-sooty
(vi) Which of the following is the characteristic of solid?
- It is packed together but does not have definite size.
- It is compact packed and has definite shape and size.
- It is loosely packed with irregular order.
- It is closely packed with uniform shape.
(vii) What is the oxidation state of Chlorine in KCIO3?
- +2
- -5
- +5
- +3
(viii) Which of the following is a sequential method of separating mixture of salt and sand?
- Evaporation, filtration and decantation
- Decantation, evaporation and filtration
- Sedimentation, evaporation and filtration
- Decantation, filtration and evaporation
(ix) Which of the following is the best apparatus for measuring accurately the volume of a given solution?
- Measuring cylinder
- Burette
- Beaker
- Conical flask
(x) The factors that affect the problem being investigated are referred to as:
- Dependent factors
- Variables
- Independent factors
- Conditions
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATION COUNCIL
CHEMISTRY FTNA 2013
 SECTION B
SECTION B
(i) The apparatus used for grinding granular chemicals in the laboratory include:
- Crucible and watch glass
- Mortar and pestle
- Pestle and pair of tongs
- Spatula and basin
(ii) The substances that can be used to extinguish fire are:
- Carbon dioxide and sand
- Carbon dioxide and sugar
- Nitrogen and sand
- Nitrogen and water
(iii) Which of the following electronic configurations are of metals?
A. 2:8:1 and 2:5
B. 2:8:2 and 2:6
C. 2:8:3 and 2:8:8:7
D. 2:8:6 and 2:8:8:7
(iv) When sugar is dissolved in water, a uniform mixture is formed. The resulting mixture is called a:
- Solute
- Solution
- Solvent
- Suspension
(v) Flammable chemicals are those which:
- Burn skin
- Catch fire easily
- Explode
- Extinguish fire
(vi) Which of the following can be classified as a renewable source of energy?
- Biomass
- Coal
- Coke
- Petroleum
(vii) The part of Bunsen burner that controls the amount of air coming in is called:
- Air hole
- Barrel
- Collar
- Jet
(ix) The simplest formula of a compound formed when combining 13g of aluminum and 17g of chlorine is:
- AlCl
- Al2Cl
- Al3Cl2
- AlCl3
(x) The second step in the scientific procedure is:
- Data collection and analysis
- Data interpretation
- Experimentation and observation
- Hypothesis formation
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATION COUNCIL
CHEMISTRY FTNA 2012
(i)All domestic utensils made of iron undergo rusting when exposed to:
- Air and fire
- Air and oil
- Air and water
- Water and oil
(ii)When a small amount of common salt is dissolved in a glass of water the mixture formed is:
- Heterogeneous
- Homogeneous
- Immiscible
- Suspension
(iii) A chemist should acquire all of the following skills except:
- Experimentatio
- Observation
- Problem identification
- Surgery

(vi) An important property of oxygen which distinguishes it from other gases is that it:
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor supports combustion
- Supports combustion but does not burn
(v) The process of chlorination in water treatment aims at:
- Forming suspension
- Killing micro-organisms
- Making syrup
- Removing bad odour
(vi) One of the following is not correct about coke being a better fuel than coal as it:
- Does not produce carbon dioxide gas
- Does not produce poisonous gas
- Has a higher heat content
- Is clean and smokeless
(vii) Class E fire can best be extinguished by using:
- Carbon dioxide
- Fire blanket
- Sand
- Water
(viii)The following is a set of apparatuses which are used for heating:
A.crucible, test tube, evaporating dish
B.evaporating dish, tongs, crucible
C.test tube, evaporating dish, tongs
D.tongs, crucible, test tube 
(ix)Which of the following methods can be used to get oil from cotton seeds?
A.Decantation
B. Distillation
C. Grinding and distillationD. Grinding followed by squeezing
(x)Which of the following apparatuses is suitable for measuring volumes of smaller quantities of liquids?
A.Beaker
B. Burette
C. Conical flask
D. Measuring cylinder
| Protons | Neutrons | Electrons | |
| A | 39 | 19 | 20 |
| B | 19 | 39 | 20 |
| C | 20 | 19 | 20 |
| D | 19 | 20 | 19 |
(vi) Which of the following warning signs is likely to appear on the bottle containing concentrated nitric acid in the laboratory?
- Corrosive
- Explosive
- Flammable
- Irritant
(vii) Saturated solution is one which:
- Contains more solute undissolved at a given temperature.
- Will take no more of solute at a given temperature.
- Contains a little solute at a given temperature.
- Has a large amount of solvent at a given temperature.
(viii)The percentage of C in C2H6 is:
- 20
- 40
- 60
- 80
(ix) Which of the following electronic configurations are of metals?
- 2:8:8:1 and 2:8:8:7
- 2:8:3 and 2:8
- 2:8:8:1 and 2:8:3
- 2:8:6 and 2:8:8:7
(x) Hard water which can be softened by boiling method contains dissolved:
- calcium carbonate
- calcium sulphate
- magnesium chloride
- magnesium hydrogen carbonate
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
FORM TWO SECONDARY EDUCATION EXAMINATION, 2007
0032 CHEMISTRY
TIME: 2 HOURS
INSTRUCTIONS
1. This paper consists of three sections A, B and C.
2. Answer all questions in spaces provided for each question.
3. Write your examination number on the top right hand corner of every page.
4. All writing must be done in “black or blue pen” except for the diagrams which must be in pencil.
5. Cellphones and calculators are not allowed in the examination room.
6. The following constants may be used:
Atomic masses: H = 1, C = 12, O = 16, and Na = 23.
This paper consists of 10 printed pages.
SECTION A (10 Marks)
1. Write down the letter corresponding to the most correct answer in the box provided for each question.
(i) When a chemist studies a substance, he/she is interested in its:
- force of attraction
- shape
- smell
- properties
(ii) When you melt a piece of iron, it undergoes:
- sublimation
- physical change
- chemical change
- combination
(iii) One isotope of an element has atomic number A and mass number M. How many neutrons are contained in the nucleus of its atom?
- M
- A
- A – M
- M – A
(iv) The total number of protons and neutrons in the nucleus of an atom is called:
- valency number
- atomic number
- molecule number
- mass number
(v) Hydrogen gas can be collected by downward delivery because:
- it burns in air with a pop-sound
- it is more soluble than air
- it is lighter than air
- it can fill balloons
(vi) The reaction that takes place when limestone CaCO3 is heated in the laboratory can be described as:
- combination
- decomposition
- replacement
- double decomposition
(vii) Which of the following warning signs is likely to be found on the bottle containing petrol?
- oxidant
- flammable
- corrosive
- irritant
(viii) The process of chlorination in water treatment aims at:
- killing micro-organisms
- removing bad odours
- forming suspension
- syrup making
(ix) Oxidation may be defined as:
- loss of hydrogen by a substance
- gain of hydrogen by a substance
- reaction in which oxygen is lost
- reaction in which electrons are increased
(x) Which of the following sets of symbols represents isotopes:

THE UNITED REPUBLIC OF TANZANIA MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
FORM TWO SECONDARY EDUCATION EXAMINATIONS, 2006
0032 CHEMISTRY
TIME: 2.00 HOURS
INSTRUCTIONS
1. This paper consists of three sections A, B and C.
2. Answer all questions on spaces provided for each question.
3. Write your examination number on the top right hand corner of every page.
4. All writing must be in black or blue pen except for diagrams which must be in pencil.
5. Cellphones and calculators are not allowed in the examination room.
6. The following constants may be used
Atomic masses: H = 1, C = 12 and O = 16, Mg = 24, S = 32
SECTION A (10 Marks)
This section consists of ten multiple choice items. You are required to answer all questions in this section.
1. Write down the letter of the most correct response in the box provided for each question.
(i) One of the following apparatuses is used to measure a fixed volume of liquids.
- Pipette
- Burette
- Measuring Cylinders
- Beaker
(ii) Coloured substances can be separated through the process called:
- Filtration
- Chromatography
- Distillation
- Sublimation
(iii) Which of the following equations is a neutralization reaction?
- Zn + Cl2 → ZnCl2
- Fe + S → FeS
- H+ + OH → H2O
- CaCO3 → CaO + CO2
(iv) Moving across the period in the periodic table:
- Electronegativity decreases
- Electronegativity increases
- Metallic property increases
- Electropositivity increases
(v) A solution of pH 1.5 is best described as:
- Weak acid
- Strong base
- Weak base
- Strong acid
(vi) A sample of chlorine gas was found to contain 75% of the isotope 35Cl17 and 25% of isotope 37Cl17. Which of the expressions below is used to calculate the Relative Atomic Mass of chlorine?
- [(35 x 75) + (37 x 25)] /100
- [(35 x 25) + (37 x 75)] / 100
- [(75 x 25) + (37 x 35)] /100
- (35 + 37)/2
(vii) Which of the following group of substances represents flammable liquids.
- Petrol, pesticides, hydrogen
- Petrol, sulphuric acid, methylated spirit
- Methylated spirit, petrol, kerosene
- Kerosene, diesel, hot water
(viii) If element M of Group I elements combines with element X of group VI, the formula of the compound formed is:
- X2M6
- MX2
- MX6
- M2X
(ix) Acids change colour of the litmus paper from:
- Blue to yellow
- Red to blue
- Red to pink
- Blue to red
(x) The untreated and treated water differ in that:
- Untreated water contains dirt while the treated contains dissolved chemicals.
- Treated water forms lather with soap while the untreated forms scum.
- Untreated water is safe for swimming while the treated can corrode the skin.
- Treated water is safe for swimming while the untreated can be harmful to health.
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF EDUCATION AND CULTURE
FORM TWO SECONDARY EDUCATION EXAMINATIONS, 2005
0032 CHEMISTRY
TIME: 2.00 HOURS.
INSTRUCTIONS
1. This paper consists of three sections A, B and C.
2. Answer all questions on spaces provided in each question.
3. Write your examination number on the top right hand corner of every page.
4. All writings must be in black or blue pen except for diagrams which must be in pencil.
5. Cell phones are not allowed in the examination room.
6. The following constants may be used
Atomic masses: H = 1, C = 12 and O = 16
SECTION A (10 Marks)
This section consists of ten (10) multiple choice items. You are required to answer all questions in this section.
1. Write down the letter of the most correct response in the box provided in each question.
(i) The states of matter are:-
- Element, gas and mixture.
- Liquid, moisture and element.
- Water, moisture and solid.
- Gas, liquid and solid.
(ii) A Substance which absorbs water/moisture from the atmosphere and forms a solution is called:
- Efflorescent.
- Amphoteric.
- Hygroscopic.
- Deliquescent.
(iii) The correct statement about metals is that:-
- React with acids to give gases.
- React with acids to give hydrogen gas.
- Have more than one valency.
- All have magnetism.
(iv) In Chemistry, experiments test:
- Data.
- Problems.
- Hypotheses.
- Observation.
(v) In any chemical change:
- Energy is not created.
- Energy is either absorbed or given out.
- Energy is created.
- Energy is neither liberated nor absorbed.
(vi) Which of the following sets of apparatus are suitable for measuring the volume of solutions?
- Burette, pipette and beaker.
- Burette, pipette and conical flask.
- Measuring cylinder, burette and pipette.
- Burette, flat bottomed flask and pipette.
(vii) The substance that can burn your skin is best described as:
- Flammable.
- Corrosive.
- Explosive.
- Toxic.
(viii) Sublimation is the process whereby
- Substances float in liquids when heated.
- Substances change directly to vapour without the liquid state when heated.
- A mixture of solid substances is separated by heating.
- An alcohol is separated from water.
(ix) Which of the following chemical species have the same number of electrons.
- K+, Ca2+, Cl- and Ar
- Cl, Be and O2
- O2-, Ca2+ and Mg2+
- Na+, Mg2+, Be2+ and Li
(x) The process of chlorination in water treatment aims at.
- Killing micro organisms.
- Syrup making.
- Forming suspension.
- Removing bad odours.
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
FORM TWO SECONDARY EDUCATION EXAMINATION, 2004
0032 CHEMISTRY
TIME: 2 HOURS
INSTRUCTIONS
1. This paper consists of three sections A, B and C.
2. Answer all questions in spaces provided for each question.
3. Write your examination number on the top right hand corner of every page.
4. All writing must be done in “black or blue pen” except for the diagrams which must be in pencil.
5. Cellphones and calculators are not allowed in the examination room.
6. The following constants may be used:
Atomic masses: H = 1, C = 12, O = 16, and Na = 23.
SECTION A
1. Write down the letter of the most correct response for each of the following questions:
(i) Which of the following reactions represent chemical change?
- Heating a solid ammonium chloride in a test tube;
- Burning candle in air;
- Adding sodium chloride solid in water;
- Putting ink on a filter paper.
(ii) Which of the following sets of symbols of elements stand for a single element?
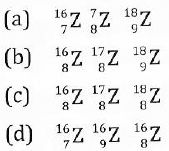
(iii) An element 'A' of electronic configuration 2:8:3 combines with an element 'B' of configuration 2:6.
The chemical formula of the compound is:
- B6A3
- A3B3
- A2B3
- A3B2
(iv) Calcium iron and calcium atom both have:
- Same physical properties
- Same number of protons
- Same number of electrons
- Same electronic configuration
(v) If a Bunsen burner flame produces much soot, which is the correct conclusion?
- The air hole is closed
- The burner gas jet is big
- The air hole is fully opened
- The gas supply is poor
(vi) The atomic number of an element is the:
- Number of protons and neutrons
- Number of neutrons
- Mass number
- Number of protons
(vii) If water does not easily form lather with soap, it is because of the presence of:
- Calcium and magnesium salts
- Calcium sulphate
- Sodium and calcium salts
- Ammonium and Magnesium salts
(viii)This mixture of substance can extinguish fire:
- Oxygen and Nitrogen
- Carbon dioxide and sand
- Carbon dioxide and Hydrogen
- Hydrogen and Neon
(ix) Which of the following sets of processes represent uses of oxygen gas?
- Welding, ice melting, magnetization
- Mountaineering, sublimation, freezing
- Glass cutting, desiccation, welding
- Diving, welding, mountaineering
(x) The reaction that takes place when NaHC03 is heated in the laboratory can be described as:
- Combination
- Decomposition
- Replacement
- Double decomposition
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
FORM TWO SECONDARY EDUCATION EXAMINATION, 2003
0032 CHEMISTRY
TIME: 2 HOURS
INSTRUCTIONS
1. This paper consists of three sections A, B and C.
2. Answer all questions in spaces provided for each question.
3. Write your examination number on the top right hand corner of every page.
4. All writing must be done in “black or blue pen” except for the diagrams which must be in pencil.
5. Cellphones and calculators are not allowed in the examination room.
6. The following constants may be used:
Atomic masses: H = 1, C = 12, O = 16, and Na = 23.
SECTION A
This section consists of multiple choice items (i) — (ii). You are required to answer all questions in this section.
1. Write down the letter of the most correct response for each question:
(i) Chemistry is one of the sciences which deals with:
- Alkalinity and basicity of substances.
- The study of body cells.
- Composition, properties and behaviour of matter.
- Chemical changes.
(ii) One isotope of an element has atomic number A and mass number M. How many neutrons are contained in the nucleus of its atom?
- M
- A
- A-M
- M-A
(iii) In the diagram T represents a:
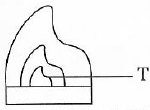
- Bunsen burner flame
- Region of unburnt gas
- Zone of complete combustion
- Gas burning
(iv) The neutral point in that pH scale is:
- 0.7
- 7
- 6
- 8.0
(v) If the element M in the group I combines with element X of group VI the formula of the compound formed is:
- MX2
- MX0
- X2M
- M2X
(vi) Group seven elements are known as:
- Alkali metals
- Transition metals
- Alkaline earth metals
- Halogens
(vii) The simplest ionic equation which summarizes the process of neutralization is:
- H+ + Nao → H2O + Na
- NaOH + CI → NaCI
- H+ + OH → H2O
- Na+CI- → NaCI
(vii) Which of the following chemical species have the same number of electrons?
- K+, Ca2+ , Cl- and Ar
- Cl-, Be and O2-
- O2-, Mg2+
- Na+, Mg2+ , Be2- and Li
(ix) A pipette is used for:
- Measuring distance of length
- Measuring specific volume of liquids
- Measuring volumes
- Heating liquid
(x) The process of chlorination in water treatment aims at:
- Killing micro-organisms
- Syrup making
- Forming suspension
- Removing bad odours
VIEW MARKING SCHEME

 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256





