Three elements, X, Y and Z, are in the same period of the periodic table. The oxide of X is amphoteric, the oxide of Y is basic and the oxide of Z is acidic. Which of the following shows the elements arranged in order of increasing atomic number?
- X, Y, Z
- Y, Z, Y
- Z, X, Y
- Y, X, Z
- X, Z, Y.
2
Which of the following electronic configurations are of metals?
A. 2:8:1 and 2:5
B. 2:8:2 and 2:6
C. 2:8:3 and 2:8:8:7
D. 2:8:6 and 2:8:8:7
3
When referring to the modern Periodic Table, the transition elements are found between:
- Group I and II
- Group I and III
- Group II and III
- Group III and IV
4
An element in the periodic table with atomic number 18 belongs to which of the following?
- Group I and period I.
- Group O and period III.
- Group III and period III.
- Group V and period IV.
- Group VII and period IV.
5
Which of the following sets of symbols represent isotopes of a single element?
-
16 8 X, 17 8 X, 18 8 X
-
16 8 Z, 17 8 Z, 18 8 X
-
16 7 P, 16 8 P, 16 9 P
-
16 7 K, 17 8 K, 18 9 K
-
16 7 U, 16 8 U, 18 10 U
6
5. (a) Study the following part of the periodic table and List down the names of all the missing elements. Table 1
| H |
|
|
|
|
|
| He |
| Li | Be | B |
|
|
| F |
|
|
|
| Al | Si | P | S | Cl | Ar |
7
Match the items in List A with the responses in List B by writing the letter of the correct response beside the item number in the answer book provided.
| LIST A | LIST B |
|
|
8
Answer all questions in section.
3. (a)Study the following portion of the periodic table with some elements represented by letters and answer the questions that follow.

(i) State how electronegativity varies from A to C and from B to D .
(ii) Write the electronic configurations of A, C2-, D and B.
View Ans9
4. (a) Consider elements with atomic number 1, 11, 12 and 17.
(i) What are the types of oxides formed by elements with atomic number 11 and 12?
(ii) Write an equation which represents a reaction between the element with atomic number 1 and 17.
(iii) Write a balanced chemical equation between the oxide of the element with atomic number 11 and aqueous solution of the compound formed in 4 (a) (ii).
View Ans10
Study the following part of the periodic table and then answer the questions that follow. Note: The letters used are not scientific symbols for the elements concerned.
Group
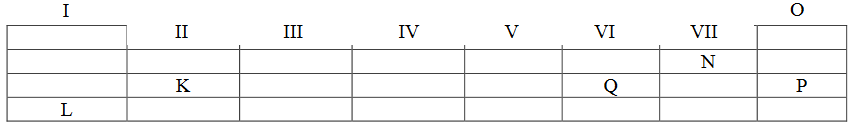
(a) Identify and write down the electronic configuration for the elements K, N, P and L.
(b) What type of bond will exist in a compound formed when Q combines with L? Write the chemical formula for the compound formed and list two chemical properties for the compound formed.
View Ans11
7.(a) Study the following Periodic Table and then answer the questions that follow.

(i)Write the collective name of elements in:
Group Il .. Group VIII 
(ii)Name the elements which are represented by the following letters:

(iii)Write the electronic configuration of the following elements:

12
3.(a) Define the following terms:
(i) Emulsions
(ii) A solution
(iii) Atom
(iv) Radical
View Ans13
(a) Study the following periodic table and then answer the questions that follow:
(i) Name elements named by the following letters: S, W, X, Z
(ii) Write the electronic configuration for the elements represented by the following letter.
View Ans14
10.Mention four chemical properties of Oxygen.
(b) Find the oxidation number of each of the underlined elements in the following: (i) KC103
(ii) 
 Use the IUPAC system to name each of the following chemical compounds:
Use the IUPAC system to name each of the following chemical compounds:
(i) Cuo (ii) CaSO4
(iii) HN03 (iv) ZnC12
15
5. Use the details given below about elements P, Q, R, S and T to answer questions (a) and (b).
| Element | Atomic number | Atomic Mass |
| P | 10 | 20 |
| Q | 11 | 23 |
| R | 12 | 24 |
| S | 13 | 26 |
| T | 14 | 32 |
(a) (i) Write down the electronic configuration of the elements represented by letters from P to T:
(i)How many neutrons are present in element Q? . 
(c)Name the type ofbonds that will be formed in the combination between the following elements: (i) Q and T
(ii) S and T
(d)Write the chemical symbol for each of the following elements:
(i)Silver
(ii)Lead .........
(iii)Manganese ......
View Ans16
9.(a) Explain why:
(i)a magnesium ion has a charge of 2+ .........
(ii)a magnesium oxide has no overall charge ..
(b) Give the name of a bond which can be formed between two oxygen atoms .......
(c) (i) State the modern periodic law
(ii)Give the special name for each of the following groups of elements in the periodic table:
Group I
Group Vll .........
(iii)Why is the atomic number a better way of identifying an element than the mass number? .
View Ans17
Below is part of the periodic table and the numbers represent atomic numbers. Study the table carefully then answer the questions that follow:
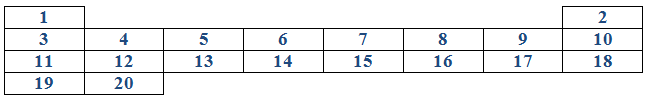
(i) Write T in the space where a noble gas in period 3 would occupy.
(ii) Write U in the space where the most active metal would occupy.
(iii) Write W in the space where the most active non-metal would occupy.
(iv) Write X in the space which would be occupied by an element in period 3 capable of forming a compound XW
(v) Write Y in group Il period 4 element.
(vi) Write Z in group VI period 3 element.
(b) Write the chemical symbols of the following elements:
(i) Argon
(ii) Sulphur
(iii)Boron
(iv)Silicon
(v)Phosphorus
(c) Write the formula of each compound formed between:
(i) Aluminium and chlorine
(ii) Potassium and oxygen
View Ans18
An atom of element X having atomic number 11 combines with an atom of element Y haying atomic number 9 to form a compound.
(a)Write the formula of the compound and state the type of bond formed in the compound.
(b) Give four properties of the compound formed in (a).
View Ans19
6. (a) Give one example in each of the following:
(i) Alkali earth metals.
(ii) Noblegases .
(iii) Transition elements.
View AnsHub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania