FORM TWO CHEMISTRY EXAM SERIES 217
FORM TWO CHEMISTRY EXAM SERIES 217
PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY SCHOOL EXAMINATION
CHEMISTRY FORM TWO
PRE-NECTA EXAMS– 2025
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 MARKS)
Answer ALL questions.
i. Which one of the following represent the chemical combination of substances result into the formation of water;
- Magnesium + oxygen → Magnesium oxide.
- Lead (II) Oxide + Hydrogen → Lead + water
- Hydrogen + Oxygen → Water
- Silver oxide + Hydrogen → Silver + Water
ii. What is so special with Francium and Fluorine compared to other elements in the periodic table?
- Francium is a liquid and Fluorine is a gas
- Francium is in group 1 and Fluorine in group 7
- Francium is in periodic 7 and Fluorine in period 2
- Francium is most electropositive and Fluorine most electronegative.
iii. A mixture of two solid substance is commonly heated in the laboratory to produce oxygen such mixture could be that of:-
- Manganese dioxide, hydrogen and magnesium
- Potassium permanganate and magnesium oxide
- Mercury (ii) oxide and hydrogen peroxide
- Potassium chlorate and manganese (iv) oxide
iv. What type of displacement is done by collecting pure hydrogen in the laboratory;
- Downward displacement of water
- Downward displacement of air
- Upward displacement air
- Upward displacement of air
v. What is kindling temperature
- A kind temperature
- Temperature out of a burning material
- The highest temperature obtained from a burning substance
- The lowest temperature at which a combustible material can catch fire.
vi. Which element will form a compound of the formular M2O3 where M is a metal?
- Aluminium and Oxygen
- Beryllium and chlorine
- Oxygen and Sodium
- Calcium and oxygen.
viii. What is so unique about a hydrogen atom on comparing it with other elements?
- It has no neutron in its nucleus
- It has a small relative atomic mass
- It forms a low density gas
- It has no exact place in the Periodic table
ix. Identify the skill not acquired during chemistry study
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
x. When one wants to light the Bunsen burner, what is the first thing to do;
- Light a match/wooden splint and hold it at the gas tap using the rubber tubing.
- Close air hole and connect the burner to the gas tap using the rubber tubing.
- Open the gas tap slowly to half way to fully open position.
- Open the hole slowly
2. Match the items in List A with their corresponding responses in List B.
| LIST A: | LIST B |
|
|
SECTION B
- (a) List down three (3) sources of natural water.
(b) Explain why water is NOT used to extinguish class E fires.
(c ) Give a reason to support the following facts
- Water is universal solvent
- Oxygen is collect over water
- Oxy-hydrogen used in welding
4. The following are atomic and ionic radii (in nm) of members of the same group of the periodic table use the information to answer the questions that follow. The letters do not represent the actual
| Element | Atomic radii (nm) | Ionic (nm) |
| A B C D E | 0.157 0.216 0.133 0.235 0.203 | 0.098 0.149 0.078 0.165 0.133 |
- Is this a group of metallic or non metallic elements? Explain your answer?
- State the element that would have lowest atomic number.
- State the element which would be the most reactive. Give a reason for your answer.
- State the element which would be the most reactive. Give a reason for your answer.
5. Gas “P” has the following properties; it is highly flammable, readily combines with other elements, readily reacts with other chemical substance and is a strong reducing agent.
- Name the gas “P”
- What is the method used to collect gas “P” in the laboratory? Give reason
- Give four (4) uses of gar “P”.
6. (a) write down the chemical formula of the following compounds
- Copper (II) nitrate _____________________________
- Sodium hydrogen carbonate _________________________
- Aluminium chloride _______________________________
(b) Write the IUPAC names of the following chemical compounds:
(i) H2SO4 ________________________________________________
(ii) HCl O3 __________________________________________________
(iii) Cu2O ____________________________________________________
7. (a) Which are the three sub – atomic particles;
- ……………………………. (ii) …………………………….. (iii) ……………………............
(b) Which sub – atomic particles from the nucleus and what is their common name?
Particles (i) ……………………………. (ii) …………………………………………
Common name …………………………………………………….
8. (a) Define:- (i) Valency (ii) oxidation state (iii) Radical.
(b) Calculate the oxidation number of the underlined elements:-
(i) Na2SO4 (ii) SO42- (iii) K2Cr2O7 (iv) NH4+ (v) MnO4-
9. (a) Define the following terms;
- Water treatment ………………………………………………………………………………………………………………………………………………………………………………
- Water purification …………………………………………………………………………………………………………………………………………………………………………………
(b) Name impurities that can be found in water?
- …………………………………………………………………………
- …………………………………………………………………………
- …………………………………………………………………………
- …………………………………………………………………………
(c) State any two methods of domestic water treatment;
(i) ……………………………………………………………………………..
(ii) …………………………………………………………………………….
SECTION C
10. (a) What is the suitable term in chemistry used for the tendency of an
electron pair towards itself? …………………………………………………………
(b) Can isolated atoms show the same behaviour? …………………………………….
(c) Which is the suitable term in chemistry used in opposite to the 7(a) and (b) behaviours above? …………………………………………………………………
………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 208
FORM TWO CHEMISTRY EXAM SERIES 208
THE OFFICE OF THE PRESIDENT, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT.
SECONDARY EXAMINATION SERIES
MARCH 2025
CHEMISTRY FORM TWO
TIME: 2:30 HRS
INSTRUCTIONS
- This paper consists of three(3) sections A, B and C with a total of ten (10) questions
- Section A carries fifteen (15) marks, section B seventy (70) marks and section C fifteen (15) marks.
- Answer ALL questions in all sections
- Write your names and stream on the top center in every page of your examination
- ALL answers should appear in this question paper in the space provided.
SECTION A (15 Marks)
(Answer all questions in this section)
1. For each of the items (i)-(x) choose the correct answer from among the given alternative and write its letter besides the item number in the answer booklet provided. (@1mark=10 marks)
(i) Chemistry is one of the sciences which deals with:
- The study of body cells
- Composition,properties and behavior of matter
- Chemical changes
- Physical changes
(ii) The process of chlorination in water treatment aims at:
- Syrup making
- Removing bad smell
- Killing micro-organisms
- Formation of suspension
(iii) The atomic number of an element is the:
- Number of protons
- Mass number
- Number of neutrons
- Number of protons and neutrons
(iv) The substance that can burn your skin is best described as:
- Flammable
- Corrosive
- Toxic
- Explosive
(v) One of the following can distinguish hydrogen gas from other gases:
- It burns and supports combustion
- It is colourless and odorless
- It gives a smell of a rotten egg
- It burns with pop sound.
(vi) The following sentences suggest that air is a mixture, except:
- Its components can be separated physically
- Its composition varies from one place to another
- Its properties depends on individual gases
- Its formation requires absorption or reabsorption of heat.
(vii) The welders prefers to use non luminous flame for their work simply because
- It is available
- it is easy to transport
- produce very hot flame
- can be made by kerosene
(viii) Spatula in the laboratory is used for scoping what types of substances?
- Liquid and gases
- solids and liquids
- Powdery and gases
- Solids and powdery
(ix) There are two particles inside the nucleus which one contributes the net change of that nucleus?
- Dalton
- Protons
- Electrons
- Neutrons
(x) Why oxygen as one of the components of air Is unique?
- It has ability to burn
- it support combustion
- it is diatonic gas
- combine with carbon dioxide
2. (a) Choose a word(s) from list B which matches the statement or phrases in list A and write its letter in the space provided.
| LIST A | LIST B |
| (i) Factors that can be manipulated to get desired results (ii) A factor that is kept constant (iii) Scientist’s best possible answer (iv) Statement of how the results related to hypothesis (v) First step in scientific method of studying a problem |
|
SECTION C (70) MARKS
3. (a) Define the term rust. (2 marks)
(b) Write down the chemical formula of rust. (2 marks)
(c) Briefly explain why iron in salt water rust faster than in fresh water? (3 marks)
(d) List down three disadvantages of rusting process in our daily life. (3 marks)
4. (a) Give out the reason why oxygen gas is normally collected by the method called downward displacement of water. (2 marks)
(b) Briefly explain how you would test the presence of the following gases in air.
- Oxygen . (2 marks)
- Carbon dioxide (2 marks)
(c) With four reasons explain why air is not termed as a compound. (4 marks)
5. The laboratory technician planned to conduct an experiment for the preparation of gas M. He decided to use a pieces of zinc metal and dilute hydrochloric acid.
- Identify gas M
- Mention six apparatus that he can use to prepare the gas M.
- Write the word equation for the laboratory preparation of gas M.
- Describe the properties of gas M which relates with its uses. Give two points.
6. (a) Explain the following terms;
- Physical change
- Chemical change
(b) Student of form two performing two simple experiments concerning changes of matter on two substances, A and B in the laboratory. In experiment number 1, student changes substance “A” from solid to liquid and in experiment number 2, student changes substance “B” by burning it to form ashes.
(i) Provide one example of each substance between A and B
(ii) What are the four differences between the changes of matter occurred in substance “A” and the changes of matter that occurred in substance “B”
7. a) Identify types of change involved in each of the following. i.e. state whether physical or chemical change,
i) Respiration
ii) Sublimation
iii) Combustion
iv) Distillation
b)Write the chemical symbols of the following elements
i) Potassium
ii) Sodium
iii) Mercury
iv) Gold
c) Write the most suitable method of separating the following mixture.
i) Air
ii) Kerosene and water
iij) Iodine and sand
iv) Syrup
8. A compound consists of 27.3% sodium, 1.2% hydrogen, 14.3% carbon a nd oxygen. Its relative atomic mass is 84
i) Calculate its empirical formula
a. Use the answer in 6(i) to find its molecular formula
ii) State the name of the compound
9. a) Elements 40W has 22 neutrons (the letter is not the actual symbols of the element). State the element's:
i) Atomic number
ii) Number of protons
iii) Electronic configuration
iv) Name of element W
b) Element G is in group 7 period 3.
i) Write the atomic number of G
ii) Write the nuclide notation of two isotopes of G
SECTION C (15) MARKS
10. (a) Scientific procedures are steps used by scientists when finding answers to scientific problems. Write the steps which correspond to each of the following.
- Kelvinia was not feeling well. Shewent to see a medical doctor at Malangali Health Center.
- The Doctor asked Kelvinia several questions about how she was feeling.
- The Doctor ordered Kelvinia’s body temperature, blood and urine sample for observation in the laboratory.
- The laboratory technician diagnosed Malaria parasite in Kelvinia’s blood.
- The doctor confirmed that Kelvinia had Malaria and prescribed medicine for her.
(b) Why is scientific procedure important? Give two points
(c) State three areas where scientific procedures are applied
FORM TWO CHEMISTRY EXAM SERIES 191
FORM TWO CHEMISTRY EXAM SERIES 191
PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM TWO
MID-TERM EXAMS – AUG/SEPT – 2024
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 MARKS)
Answer ALL questions.
i. Which one of the following represent the chemical combination of substances result into the formation of water;
- Magnesium + oxygen → Magnesium oxide.
- Lead (II) Oxide + Hydrogen → Lead + water
- Hydrogen + Oxygen → Water
- Silver oxide + Hydrogen → Silver + Water
ii. What is so special with Francium and Fluorine compared to other elements in the periodic table?
- Francium is a liquid and Fluorine is a gas
- Francium is in group 1 and Fluorine in group 7
- Francium is in periodic 7 and Fluorine in period 2
- Francium is most electropositive and Fluorine most electronegative.
iii. A mixture of two solid substance is commonly heated in the laboratory to produce oxygen such mixture could be that of:-
- Manganese dioxide, hydrogen and magnesium
- Potassium permanganate and magnesium oxide
- Mercury (ii) oxide and hydrogen peroxide
- Potassium chlorate and manganese (iv) oxide
iv. What type of displacement is done by collecting pure hydrogen in the laboratory;
- Downward displacement of water
- Downward displacement of air
- Upward displacement air
- Upward displacement of air
v. What is kindling temperature
- A kind temperature
- Temperature out of a burning material
- The highest temperature obtained from a burning substance
- The lowest temperature at which a combustible material can catch fire.
vi. Which element will form a compound of the formular M2O3 where M is a metal?
- Aluminium and Oxygen
- Beryllium and chlorine
- Oxygen and Sodium
- Calcium and oxygen.
viii. What is so unique about a hydrogen atom on comparing it with other elements?
- It has no neutron in its nucleus
- It has a small relative atomic mass
- It forms a low density gas
- It has no exact place in the Periodic table
ix. Identify the skill not acquired during chemistry study
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
x. When one wants to light the Bunsen burner, what is the first thing to do;
- Light a match/wooden splint and hold it at the gas tap using the rubber tubing.
- Close air hole and connect the burner to the gas tap using the rubber tubing.
- Open the gas tap slowly to half way to fully open position.
- Open the hole slowly
2. Match the items in List A with their corresponding responses in List B.
| LIST A: | LIST B |
|
|
SECTION B
- (a) List down three (3) sources of natural water.
(b) Explain why water is NOT used to extinguish class E fires.
(c ) Give a reason to support the following facts
- Water is universal solvent
- Oxygen is collect over water
- Oxy-hydrogen used in welding
4. The following are atomic and ionic radii (in nm) of members of the same group of the periodic table use the information to answer the questions that follow. The letters do not represent the actual
| Element | Atomic radii (nm) | Ionic (nm) |
| A B C D E | 0.157 0.216 0.133 0.235 0.203 | 0.098 0.149 0.078 0.165 0.133 |
- Is this a group of metallic or non metallic elements? Explain your answer?
- State the element that would have lowest atomic number.
- State the element which would be the most reactive. Give a reason for your answer.
- State the element which would be the most reactive. Give a reason for your answer.
5. Gas “P” has the following properties; it is highly flammable, readily combines with other elements, readily reacts with other chemical substance and is a strong reducing agent.
- Name the gas “P”
- What is the method used to collect gas “P” in the laboratory? Give reason
- Give four (4) uses of gar “P”.
6. (a) write down the chemical formula of the following compounds
- Copper (II) nitrate _____________________________
- Sodium hydrogen carbonate _________________________
- Aluminium chloride _______________________________
(b) Write the IUPAC names of the following chemical compounds:
(i) H2SO4 ________________________________________________
(ii) HCl O3 __________________________________________________
(iii) Cu2O ____________________________________________________
7. (a) Which are the three sub – atomic particles;
- ……………………………. (ii) …………………………….. (iii) ……………………............
(b) Which sub – atomic particles from the nucleus and what is their common name?
Particles (i) ……………………………. (ii) …………………………………………
Common name …………………………………………………….
8. (a) Define:- (i) Valency (ii) oxidation state (iii) Radical.
(b) Calculate the oxidation number of the underlined elements:-
(i) Na2SO4 (ii) SO42- (iii) K2Cr2O7 (iv) NH4+ (v) MnO4-
9. (a) Define the following terms;
- Water treatment ………………………………………………………………………………………………………………………………………………………………………………
- Water purification …………………………………………………………………………………………………………………………………………………………………………………
(b) Name impurities that can be found in water?
- …………………………………………………………………………
- …………………………………………………………………………
- …………………………………………………………………………
- …………………………………………………………………………
(c) State any two methods of domestic water treatment;
(i) ……………………………………………………………………………..
(ii) …………………………………………………………………………….
SECTION C
10. (a) What is the suitable term in chemistry used for the tendency of an atom to attract an
electron pair towards itself? …………………………………………………………
(b) Can isolated atoms show the same behaviour? …………………………………….
(c) Which is the suitable term in chemistry used in opposite to the 7(a) and (b) behaviours above? …………………………………………………………………
………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 183
FORM TWO CHEMISTRY EXAM SERIES 183
THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY FORM TWO PRE- MOCK EXAMINATION
CODE 032
TIME: 2:30 HOURS
INSTRUCTIONS.
- This paper consists of section A, B and C with the total number of ten(10) questions
- Answer all questions in each section
- Section A carries (15) marks, section B (70) marks and section C carries (15) marks
- All writing must be in blue/black ink except drawing which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A (15 Marks)
Answer all questions in this section
1. For each of the items (i) – (x) choose the correct answer from among the given alternatives and write its letter beside the item number in the answer sheet provided
- Which of these is NOT a branch of chemistry?
- Organic Chemistry
- Biochemistry
- Astrology
- Physical Chemistry
- A student mixes two clear liquids and observes a bright yellow solid form and settle at the bottom. This is likely evidence of:
- Physical change
- Chemical change
- Evaporation
- No change occurring
- What's the first step if a chemical splashes into a student's eyes in the lab?
- Rub the eyes vigorously
- Get the teacher's attention
- Go directly to the nurse
- Flush eyes with water at an eyewash station
- Which of these is a compound?
- Copper (Cu)
- Saltwater
- Carbon Dioxide (CO2)
- Air
- The main component of the air we breathe is:
- Oxygen
- Carbon dioxide
- Nitrogen
- Helium
- What three conditions are needed for fire to burn?
- Fuel, water, air
- Fuel, oxygen, heat
- Heat, light, water
- Wind, oxygen, fuel
- A Bunsen burner's hottest flame is achieved when it is:
- Tall and yellow
- Roaring and blue
- Short and orange
- Flickering and red
- A glowing splint will relight when placed in a container of pure oxygen. This shows that oxygen:
- Supports combustion
- Creates water
- Is a product of combustion
- Is not flammable
- The characteristic "pop" sound in a hydrogen gas test indicates:
- Hydrogen forms water quickly
- Hydrogen is flammable
- Hydrogen is lighter than air
- Hydrogen is odorless
- Most of an atom's mass is concentrated in the:
- Nucleus
- Protons
- Electrons
- Electron shells
2. Match the items that tend to rust with the method for preventing it.
| Column A | Column B |
|
|
SECTION B: 70 MARKS
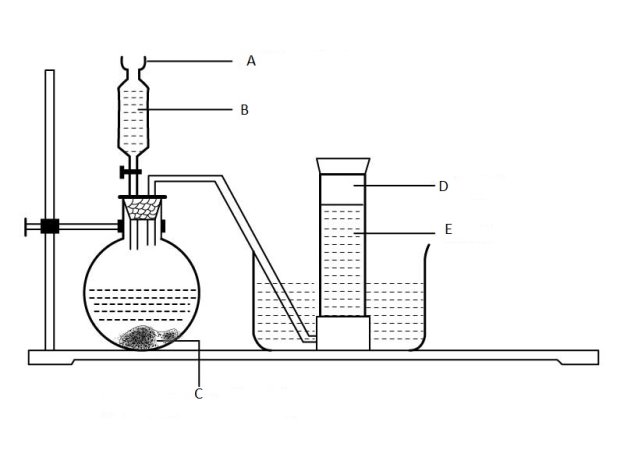
- The diagram below shows the relationship between the physical states of matter. Study it and answer the questions that follow.
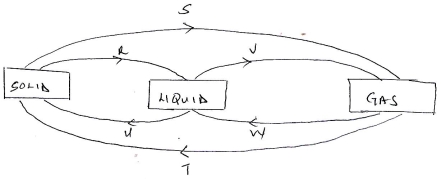
- Identify the process R,V,W and U
- Name three substances which can undergo the process represented by process S and T.
- (a) The table below shows liquids that are miscible and those that are immiscible
Liquid L3 L4
L1 Miscible Miscible
L2 Miscible immiscible
Use the information given to answer the questions that follow.
- Name the method that can be used to separate L1 and L3 from a mixture of the two.
- Draw and name an apparatus that can be used to separate a mixture of L2 and L4.
- Give two reasons why most Laboratory apparatus are made of glass.
- Name three sources of heat beside Bunsen burner in the laboratory.
- a) Draw a labeled diagram of a non-luminous flame produced by the Bunsen burner
b) State two reasons why a non-luminous flame is preferred for heating.
c) After use a non-luminous flame should be put off or adjusted to a luminous flame. Explain.
- (a) Name three apparatus that are used to measure accurate volume of liquids.
- Distinguish between an element and a compound and give an example of each.
- By use of a diagram between a residue and a filtrate.
- (a) Name the method you would use to separate the following mixtures.
- Sand and ammonium chloride.
- Oil and Water.
- Kerosene and crude oil
- Salt and water.
- Describe how you would separate a mixture of salt, sand and iodine into different components.
- (a)State the functions of the following apparatus as used in the laboratory.
- Spatula
- Pine-clay triangle
- Wire gauze
b) Draw and state the use of a deflagrating spoon.
(c )State the two causes of accidents in a Chemistry laboratory. (2mks)
- (a)Define the following terms
- Solvent extraction
- Hydrated salt
- Saturated Solution
- State two functions of a fume cupboard as found in a chemistry laboratory.
(c)Explain the differences between solid and gaseous states using the theoretical model of matter in terms of the Kinetic theory.
SECTION C: 15 Marks
10.
I. (a) Common table salt is contaminated with copper (ii) oxide. Explain how Pure sodium Chloride can be obtained from the mixture.
- The table below gives information on some substances. Use it to answer the question that follows.
| Substances | Melting Point oC | Boiling point oC | Solubility in water |
| A | -177 | 78.5 | Very soluble |
| B | -23 | 77 | insoluble |
| C | -219 | -183 | Slightly soluble |
| D | -78 | -33 | soluble |
- Which substance has the
- Lowest melting point
- Highest boiling point
- Which letters represents a substance that is a gas at room temperature?
- Which is a liquid at room temperature and when mixed with water two layers would be formed.
- Which substance dissolves in water and could be separated from the solution by fractional distillation.
- a) Give the symbols of the following elements
- Sodium
- Calcium
- Potassium
- Name the elements presents in the following compounds
- Zinc sulphide
- Sodium oxide.
FORM TWO CHEMISTRY EXAM SERIES 173
FORM TWO CHEMISTRY EXAM SERIES 173
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
MID TERM ONE – MARCH-2024
CHEMISTRY FORM TWO
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 Marks)
Answer all questions in this section
- For each of the following items (i-x).Choose the correct answer from given alternatives and writer its letter besides the item number in the box provided.
- What scientific procedure follows after data interpretation
- Observation
- Hypothesis
- Conclusion
- Experimentation
- The teacher was demonstrating experiment by dissolving Sodium Chloride in water until the solute was not dissolving anymore. What solution was forms at the end?
- Saturated
- Unsaturated
- Super saturated
- Suspension
- A large percentage of air is composed of
- Nitrogen
- Nobel bas
- Carbon dioxide
- Oxygen
- How do chemist refer mixture of water and milk
- Emulsion
- Suspension
- Misable solution
- Immisable solution
- We boil drinking water in order to:
- Remove oxygen
- Remove impulses
- Make it tasteless
- To kill micro-organisms
- The best way of prevent rusting of fragile instrument like camera is
- By using silica gel
- By using ethanol
- By galvanization
- By using oil
- The net charge inside nucleus of an atom is contributed by
- Protons
- Neutrons
- Electrons
- All nucleus
- Why is oxygen a unique component of air?
- Support combustion
- It is diatomic gas
- It forms the largest part of the air
- It has large density
- Which condition is necessary for the nails to rust?
- Oxygen and moisture
- Carbon and oxygen
- Carbon dioxide and oxygen
- Oxygen and nitrogen
- Match each item in LIST A with corresponding answer in list B and write letter of correct answer besides the item number
| LIST A | LIST B |
| (i) Inflating weather balloons |
|
| (ii) Manufacturing of Ammonia | |
| (iii) Manufacture or margarine | |
| (iv) Production of Oxy-hydrogen flame | |
| (v) Manufacture of Hydrochloric acid |
SECTION B – 85 marks
- (a) A laboratory technician instructed form two student to dissolve sodium chloride in distilled water. Give two reasons to state whether a mixture or compound was formed.
(b) Which method can be used useful in separating each the following components?
- Pure water from tea
- Oil from mixture of oil and water
- Ethanol from mixture of water and ethanol
- Salt from sea water
(c) Which change of state of matter is applied in the following process?
- Metallurgy
- Drying materials
- (a)Identify type of changes involve in each of the following processes either physical or chemical
- Respiration
- Sublimation
- Combustion
- Distillation
(b) Write the chemical symbols of the following
- Potassium
- Sodium
- Mercury
- Gold
(c) Give examples of apparatus made up of;
- Porcelain
- Glass material
- (a) Madam Jenipher was preparing Mandazi on a frying pan. Accidentally the pan toppled and huge fire spread on the kitchen floor.
- Which fire extinguisher would be suitable for putting off the fire
- Why water is not suitable to put off the fire?
(b) Give reason to support each of the following
- Commodities like handbags and camera for sale are packed with silica gel
- When iron sheet is exposed to wet and air for longtime they turn to reddish brown color.
- If clothes worn by your friend catch fire, cover them with a fire blanket.
- Mr Mwageni decided to prepare gas M in the Laboratory. He used Hydrogen peroxide as one of the reagent to prepare the gas.
- Identify gas M
- Mention all apparatus used to prepare gas M
- Write word equation for preparation of Gas M
- state uses of gas M
- (a) The chemistry teacher wanted to label container in laboratory with correct warning sign. Help him label the following containers
- Rat poison
- Methylated spirit
- Hydrogen peroxide
- Concentrated Suphuric acid
- Cooking gas
(b) Outline the first aid procedures to a person who has fainted
- (a) Define the following
- water
- water cycle
(b) List four importance processes involved in circulation of water
(c) Identify two commercial use of water
- (a) Name two reagents used to prepare Hydrogen gas in Laboratory
(b) Write word equation for reaction above
(c) Describe chemical test for hydrogen
(d) Explain why hydrogen is not used in balloons nowadays
(e) Give two uses of hydrogen
- (a) What is a flame
(b) Differentiate between the two types of flames
(c) What factors do you consider when choosing heat source in the laboratory?
FORM TWO CHEMISTRY EXAM SERIES 165
FORM TWO CHEMISTRY EXAM SERIES 165
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM TWO
MID-TERM EXAMS – AUGUST – 2023
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 MARKS)
- For each of items (i) – (x) choose the correct answer among alternatives given.
- Factors in an experiment that can be manipulated to get desired results is called
- Controlled variable
- Manipulated variable
- Dependent variable
- Independent variable
- What might happen to a careless pupil in the laboratory
- He may get injures
- He will not achieve in studies
- He will cause loss of things
- Nothing will happen
- The formation of water when hydrogen bums in Oxygen shows that
- Hydrogen is an element
- Air supports combustion
- Water is Oxide of hydrogen
- Water is an product of combustion
- Which criterion has been used to assign the symbol Ag of the element?
- The use of an English name to the element
- The use of a Latin name is the element
- Capitalizing first letter, then third letter small from the Latin name.
- Capitalizing first latter then third small from an English name.
- The moving solvent in the chromatographic column is called _________
- Mobile phase
- Stationary phase
- Analyte
- Chromatogram
- What does it indicate when the match head on base of Bunsen Burner flame does not ignite or burn
- The gas is not enough at that point
- The gas is not burnt at that point
- The match head easily bums when subjected to friction
- The match head has not be pushed on the flame from outside
- Why is covering iron or steel interferes with the rusting of iron?
- It cuts off oxygen
- It prevents direct contact with water
- It prevents contact of iron or steel with oxygen and with water
- It extends the time required by iron to rust
- The property of Oxygen that can be used for its test is
- It is colorless and odorless
- It is lighter than air
- It relights a glowing splint
- It is slightly soluble in water
- A certain liquid dissolves copper (II) Sulphate to form a blue solution, this liquid is likely to be
- Hydrochloric acid
- Liquid Oxygen
- Nitric acid
- Water
- Isotopes of Bonon appear as 10B of 20% abundance. What would be its average relative atomic mass?
- 10.80
- 10.08
- 10.88
- 10.82
- Match the description in LIST A with the correct response from LIST B by writing the letter of the correct response in the space provided.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section
- (a)A stone is said to be a good example of matter Give two reasons to support this fact.
(b)With reasons explain why it is necessary to have the following in Laboratory
- Laboratory door open outward
- Laboratory floor is rough and never polished
- Bucket of sand is found near petrol station
- (a)A student aimed to prepare gas G by using moderate reactive metal with a dilute acid. By using the information given above, answer the following questions.
- What is the name of gas G?
- What is the chemical test that distinguishes this gas from other gases
- With two reasons, state the correct means of collecting the gas?
- Write the balanced chemical equation for the reaction
(b)Juma wanted the above reaction to go fast because wants to utilize in venous industrial uses. You as a chemist provide him four means that can be used to make reaction faster.
- (a)T and K are elements found in the periodic table. The atomic number of T is 16 and that of K is 19
- In which group and period of periodic Table does element T and K appear?
- Write the chemical formula of a compound formed between T and K
(b)(i) Which particles are atoms of the same element in the list of the particles given below?
![]()
(ii) Why can’t neon react with sodium?
- (a)Define molecular and Empirical formula?
(b)A compound contains 20% by mass mg, 26% of sulphur and Y% of Oxygen
- Find empirical formula of the compound
- Find its molecular formula.
- Study the periodic table below
| I VIII | |||||||
| A | II III IV V VI VII | B | |||||
| C |
| D |
|
|
|
| E |
| F |
|
|
|
| G | H |
|
|
| J |
|
|
|
|
|
|
Use the letters shown in the periodic table above to indicate
- Element with Zero valence
- The lightest atom
- The alkali, earth metal
- An element with the electronic configuration of 2:8:1
- Give the names of element represented by the mentioned letters, A, B, C and D
- give the name of J on an element
- the gaps left out by _____ periodic table is now filled by discovered _______, ______, _____ and the artificially made elements
(b)Write valence of following radicals
- Ammonium
- Magnesium sulphate
- (a)List down three (3) sources of water
(b) Explain why water is not used extinguish class E fires
(c)Give a reason to support the following facts
- Water is universal solvent
- Oxygen is collected over water
- Oxy-hydrogen is used in welding
- (a)Mariam was preparing food for her family using hot oil in frying pam. Accidentally the Pam tripped over and huge fire spread over her kitchen floor.
- Mention two fire extinguishers which would be appropriate to like when trying to put out the fire?
- Which fire extinguisher would be dangerous to use.
(b)Mention three conditions for fire to start
- (a)Briefly explain any five methods of preventing rusting
(b)A certain pink colored compound is heated to form blue colour. When water is added to sample, it changed back to pink
- What change is demonstrated by the compound
- Explain reason for your answer.
FORM TWO CHEMISTRY EXAM SERIES 154
FORM TWO CHEMISTRY EXAM SERIES 154
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM TWO
TERMINAL EXAMS-MAY– 2023
033
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carry seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
- For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
(i) What is the best way of preparing hydrogen gas in the laboratory?
- By reacting strong metals and dilute acids.
- By reacting metals and acids.
- By reacting moderate metals and concentrated acids.
- By reacting moderate metals and dilute acids.
- By reacting strong metals and strong acids.
(ii)Consider the following reagents:
- H2O2
- H2O
- MnO4
- .MnO2
Which reagents are involved in the preparation of oxygen gas in the laboratory?
- I and 2
- 3 and 4
- CI and 3
- 2 and 3
- I and 4
(iii)A good fuel is the one which has
- high speed of continuous energy supply.
- high energy value supplied.
- low carbon dioxide supplied.
- high carbon dioxide production.
- high content of non-combustible material.
(iv) Which of the following pairs constitute the best methods for treating and purifying water?
- Chlorination and aeration
- Chlorination and decantation
- Chlorination and filtration
- Chlorination and sedimentation
- Chlorination and distillation
(v) A certain liquid dissolves copper (Il) sulphate to form a blue solution. This liquid is likely to be:
- Hydrochloric acid
- Liquid oxygen
- Nitric acid
- Water
- Lime water
(vi) Isotopes are atoms of the same element that have different:
- Atomic number
- Electron arrangement
- Mass number
- Protons
- Neutrons
(vii) An important property of oxygen which distinguishes it from other gases is that it:
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor supports combustion
- Supports combustion but does not burn
- Reacts with metals and Non-Metals
(viii) Which of the following electronic configurations are of metals?
A. 2:8:1 and 2:5
B. 2:8:2 and 2:6
C. 2:8:3 and 2:8:8:7
D. 2:8:6 and 2:8:8:7
E. 2:8 and 2:8:7
(ix) When a burning fuel produces blue colour it means there is:
- adequate supply of oxygen with production of soot
- inadequate supply of oxygen with production of more heat.
- inadequate supply of oxygen with production of soot.
- adequate supply of oxygen with production of more heat.
- Insufficient fuel
(x) The process of coating iron or steel with zinc is known as:
- zinc painting.
- alloying.
- tin plating.
- galvanization.
- Painting.
2. Match the description of a gas in list A with corresponding gas in List B and write the answer in the space provided.
| LIST A | LIST B |
|
|
SECTION B. 70 MARKS- ANSWER ALL QUESTIONS IN THIS SECTION
3. (a) Define the following terms as used in chemistry.
- Atom
- Atomic number
- Mass number
- Radicals
- Isotopes
(b) The table below shows the relative atomic masses and the percentage abundance of the isotopes L1 and L2 of element L.
|
| Relative atomic mass | % abundance |
| L1 | 62.93 | 69.09 |
| L2 | 64.93 | 30.91 |
Calculate the relative atomic mass of element L.
4.(a) The grid given below represents part of the periodic table. Study it and answer the questions that follow. (The letters do not represents the actual symbol of the elements)
- What name is given to the group of elements to which C and F belong?
- Compare the atomic radius of element C and F. explain
- Which letter represents the element that is the least reactive?
- What type of bond is formed when B and E react? Explain
- Write the formula of the compound formed when element D and oxygen gas react.
- On the grid, indicate with a tick the position of element G which is in the third period of the periodic table and forms G3- ions
(b) The table below gives the number of electrons, protons and neutrons in substances X, Y and Z.
Study it and answer the questions that follow.
| Substance | electrons | protons | neutrons |
| X | 10 | 10 | 10 |
| Y | 10 | 8 | 10 |
| Z | 8 | 8 | 8 |
(i) Which element represents and Ion
(ii) Which of the substances are isotopes? Give a reason.
5.(a) An organic compound P consist of 52.2% of carbon, 13% of hydrogen and 34.8% of oxygen. The vapour density of P is 23. Calculate the molecular formula of the compound P and write possible isomer(s) from the molecular formula determined.
(b) Hydrogen peroxide breaks down slowly to form water and oxygen; the reaction can be speed up by using a catalyst.
- How does the catalyst speed up the rate of reaction?
- Name a possible catalyst that can be used to speed up the reaction.
- Show that the catalyst always remains unchanged at the end of the reaction.
6.(a) Briefly explain the following
(i) Nitrogen gas is a necessary component of atmosphere
(ii) Nitrogen I Oxide is also called a Laughing gas
(iii) When carbon dioxide is passed through lime water, it turns milky
(iv) Things made of iron rust faster in coastal towns like Dar es salaam than in areas like Dodoma.
(v) Laboratory should have large windows
7.Using a well labelled diagram, explain how simple distillation can be used to separate a mixture of water and alcohol.
8. (a)What properties of hydrogen gas that made it to be used in the following?
i. As a fuel
ii. To fill weather balloons
iii. Manufacture of hydrochloric acid
iv. Manufacture of margarine
(b) Environmental pollution in most of rural areas in Tanzania is caused by using charcoal and firewood as a fuel. What will be the two alternative source of energy, they supposed to use for environmental conservation? (give reason for each)
(c) Why fossil fuels are referred to as non-renewable energy resources? (Give two reasons)
9. (a) (i) A table salt is a common name for the compound with the formula NaCl.
Write the systematic name for the table salt
(ii) Briefly describe why molecular formula better preferred than empirical formula is
(b) A compound consists of 27% sodium, 16.5% nitrogen and 56.5% oxygen by mass. If its molecular mass is 85g, find:
(i) Its empirical formula
(ii) Molecular formula
10. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
FORM TWO CHEMISTRY EXAM SERIES 143
FORM TWO CHEMISTRY EXAM SERIES 143
PRESIDENT OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSESSMENT
032 CHEMISTRY FORM TWO
MID-TERM EXAMS MARCH – 2023
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in the spaces provided
- All writing must be in black or blue ink except diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- The following atomic masses may be used: H= 1, N= 14, O= 16, S=32, Ca=40.
SECTION A
1. For each of the items (i) – (x) Choose the correct answer from among the given alternatives and write its letter on answer booklet
- Factors in an experiment that can be manipulated to get desired results are called
- Controlled variable
- Manipulated variable
- Dependent variable
- Independent variable
- One of the following apparatus is used to measure fixed volume of a liquid
- Pipette
- Burette
- Measuring cylinder
- Beaker
- A student who gets burnt accidentally in chemistry laboratory; would be given one of the following first Aid
- Antibiotic solution
- Nitric acid
- Petroleum Jelly
- Potassium Permanganate
- What is the name given to the constant temperature of a substance changing its state from liquid to solid
- Melting point
- Boiling point
- Freezing point
- Sublimation point
- Point out the odd man out in the following group of elements
- Zinc, sulphur sodium
- Copper, sodium, iron
- Aluminum, sodium, zinc
- Sodium, zinc, copper
- Jenipher wanted to obtain pure water from dirt water, which process did she use;
- Evaporation and sublimation
- Evaporation and crystallization
- Evaporation and condensation
- Evaporation and decantation
- The choice of source of heat depend on the
- Color of the flame
- Quantity of heat produced
- Substance to be bummed in air
- Types and shape of flame
- When Anna was preparing food, the frying pan got fire. What type of fire extinguishers would you advice her to use.
- Carbon dioxide
- Fire blanket
- Sand
- Water
- An important property of Oxygen that distinguishes it from other gases is that;
- Burns and Support combustion
- Bum but does not support combustion
- Neither burns or support combustions
- Support combustion
- The chemical used to test for the presence of water in a substance is
- Cobalt II Oxide
- Cobalt III Oxide
- Cobalt Chloride
- Copper II Chloride
2. (a)Match the property in List A with the term in List B by writing the letter of correct letter on space provided
| LIST A | LIST B |
|
|
(b)Fill the blank below with correct answer
- Water is Oxide of __________ and ________
- The catalyst used in preparation of Oxygen is ___________
- Why is hydrogen used in filling balloons _____________
- The reagents used to prepare hydrogen in laboratory are __________ and _____
- The process of circulation of water is atmosphere is called ___
SECTION B
3. Care “P” has the following properties; it is lightly flammable readily combiner with other elements readily reacts with other chemical substance and is a strong reducing agent.
- Name the gas “P”
- What is the method used to collect gas “P” in the laboratory? Give reason
- Give 4 uses of gas “P”
4. (a)Write the names of chemical substances used to test the presence of water
(i) _______ and (ii) _____
(b)Write the examples in which water occur are
- Solid
- gas
(c)Name chemical substance used in laboratory preparation of hydrogen gas.
5. (a)Write a word chemical equation to shoo the deco portion of hydrogen peroxide in the presence of manganese (iv)Oxide
(b)Why Oxygen gas is collected over water?
(c)Respiration and burning are similar process in some ways and difference process in other ways. Give two differences between them
6. Consider the diagram below USED to prepare hydrogen gas and then answer the questions that follow.
|
|
Label parts
- A _________
- B ___________
- C ____________
- D _____________
- F. ___________
7. (a)What is a fuel
(b)Describe six qualities of a good fuel
8. (a)State two importance of studying chemistry
(b)Mention two items used in each of the following categories that are made though application of chemistry
- Agriculture
- Pharmaceuticals
- Household items
- Food and beverage
- Transport
9. (a)State three components of fire Triangle
(b)Mention three conditions for rusting
(c)Rusting occur rapidly in Dar – es – Salaam than in Dodoma Elaborate
10. (a)What is a flame
(b) Write five differences between luminous and non-Luminous flame
FORM TWO CHEMISTRY EXAM SERIES 136
FORM TWO CHEMISTRY EXAM SERIES 136
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY FORM TWO
TIME: 2 HOURS NOVEMBER 2022
INSTRUCTIONS
1. This paper Consist of sections A, B and C with total of 10 questions
2. Answer all questions in both sections
3. All writings should be in blue/black ink
4. All diagrams should be drawn in pencil
5. Write your assessment number at the top right corner
SECTION A (15 Marks)
1. For each of the items (i)-(x), choose the correct answer from among the given alternative and write its letter in the box provide.
(i) At room temperature in which substances are particles furthest apart?
A. Water
B. Sodium chloride
C. Magnesium
D. Hydrogen
E. Hydrochloric acid
(ii)In the molecules CH4, HCI, and H20 which atoms use all of their outer shell ![]() electrons in bonding?
electrons in bonding?
A. C and C!
B. C and H
C. a and H
D. H and O
E. C and O
(iii) In the laboratory one spoon of sugar was mixed with 200cm3 of water in a beaker to form .mixture
A. Suspension
B. Homogeneous
C. Emulsion![]()
D. Miscible
E. Heterogeneous
(iv) The part of the Bunsen burner which controls the amount of air to support burning of the gas is
A. Jet
B. Air hole
C. Chimney
D. Collar
E. Gas inlet
(v) A certain mixture contained 280cm3 of alcohol and 300cm3 of water in the round bottomed flask, a form I student separated this mixture by fractional distillation process. Why?
A. The two components have approximately the same volume
B. The volume of water is less than the volume of alcohol
C. The two component differ in boiling point
D. The two component differ in volume
E. Both are liquids at room temperature![]()
(vi) Which change of matter occurs when water is kept at (zero) OOC?
A. Water and vapour
B. Liquid to gas
C. Liquid to solid
D. Water to steam
E. Water to ice
(vii) A non - luminous flame is suitable for heating because
A. It is very noisy
B. It is very hot
C. It has no soot
D. It is very large
E. It produce light
(viii) Calcium ion and calcium atom both have the same
A. Size
B. Physical properties
C. Number of protons
D. Electronic configuration
E. Charge
(ix) Class B fire can be extinguished by using each of the following
A. Oxygen and nitrogen
B. Carbondioxide and sand
C. Carbondioxide and water
D. Sand and water
E. Hydrogen and neon
(x)..........................has the highest percentage abundance in air
A. Nitrogen
B. Oxygen
C. Noble gas
D. Water vapour
E. Carbondioxide
2. Choose a word(s) from LIST B which matches the statement in LIST A and write its letter in the table provided below.
| LIST A | LIST B |
|
|
SECTION B: (70 MARKS)
Answer all questions in the space provided for each question
3. (a) There is a room at Mtakuja secondary school, having only the following features
- Large windows
- One door open inwards
- Slippery floor
- No water supply
Does the room qualify to be used as a chemistry laboratory? Give three reasons
(b) Calculate the molar mass of
(i) A1203
(ii) Ca(OH)2
(iii) C02
4.(a) ![]()
(b)Element T has 19 electrons and mass number of 39, what is the; (i) Atomic number of element T?
(ii) Number of neutrons of element T?.
(iii) Number of protons of element T?
(iv) Write the nuclide notation of element T.....
(v) Give the name of element T....
(c) ![]() 204J 206K 207L and AM are isotopes of element Q whose abundances are 2%, 24%, 22% and 52% respectively. Calculate the mass number A of an isotope M, given that the relative atomic mass of element Q is 207
204J 206K 207L and AM are isotopes of element Q whose abundances are 2%, 24%, 22% and 52% respectively. Calculate the mass number A of an isotope M, given that the relative atomic mass of element Q is 207
(d)A form Il student from Pwani secondary school conducted an experiment as follows:He boiled a hydrated copper (Il) sulphate blue solution in a crucible until all water evaporated. Explain the changes observed during experiment.
5. (a) Mr Juma was passing through in the laboratory and found a beaker on top of a table. He picked up the beaker with his bare hand, accidentally the beaker cut his finger since it was broken. Suggest four possible items of the first aid and their function to help Mr Juma.
| Items | Function |
|
|
|
(b) Give any three reasons to support that sodium chloride is a compound but Air is a mixture of gases.
| Sodium chloride as a compound | Air as a mixture of gases |
|
|
6. (a) Write the name of the following compounds
(i) (NH4)2C03
(ii) N204
(iii) FeC13
(b) Calculate the oxidation state of the underlined element in the given compound
(i) KC103
(ii) PbO2
(c) Write the chemical formula for the following compound
(i) Calcium nitrate
(ii) Zinc hydroxide
(iii) Copper (I) oxide
7. (a) The diagram below shows the position of some elements in the periodic table. Study it and answer the questions below Note: The letters given are not the actual symbols of elements

(i) Identify the most reactive non-metal......................................................................
(ii) Give the formula of only one stable ion with an electronic configuration of 2:8
(iii) Which letter represents an element with a positively charged ion............
(iv) Explain the trends of atomic radii from X to U...........................
(v) Identify elements represented by letters; W, Z, X and Y
- W................................
- Z....................................
- X.....................................
- Y.......................................
(vi) State group and period of element X
| Element | Group | Period. |
|
|
|
|
(vii) Explain why element V is stored under paraffin or Kerosine?
![]() (b) A large number of Tanzania society are using firewood and charcoal as a source of fuel for domestic uses because it is very cheap and readily available but it has a negative effect in the environment. Assess the effect of such fuel in the environment
(b) A large number of Tanzania society are using firewood and charcoal as a source of fuel for domestic uses because it is very cheap and readily available but it has a negative effect in the environment. Assess the effect of such fuel in the environment
(c) (i) Coal and Petroleum are said to be a natural source of fuel. Explain why they are termed as non -renewable source of energy?
(ii) State the law of conservation of energy
(iii) Petrol acid kerosene are liquid fuel. When burn they release energy, identify what type of energy they produce? (Give any two points)
8. (a) Organic compound M composed of 52.2% of carbon, 13% of hydrogen and the rest is oxygen. If the vapour density of compound M is 23 (i) Find the percentage of oxygen in a compound M
(ii)Calculate the empirical formula of compound M
(iii) Calculate the molecular formula of compound M
9. (a) Draw a well labelled diagram of laboratory preparation of a colorless gas which support combustion at room temperature (without heating)
(b) State the property that support hydrogen gas to be used in
(i) Production of margarine
(ii) Making oxy — hydrogen flame.
(iii) Production of water gas
(iv) Filling of weather balloons......................
SECTION C: (15 MARKS)
10. (a) Four experiment were conducted using chemical A, B, C and D on different materials, the results were given as indicated below. You are required to write the correct terminology which suits each of the results given and draw the chemical warning signs which goes with it
(i) When chemical A was poured on the wool it completely damage the surface of the wood
(ii)When chemical B was poured on the small fire, it found burst and turning a bigger fire
(iii) When chemical C was brought near the skin of some one, he started to ![]() complain that his skin was itching
complain that his skin was itching
(iv) When chemical D was exposed on air sudden blust accompanied with light occurred.
| Terminology | Terminology | Chemical warning sign |
|
|
|
(b) List down any two importance of changing states of matter in our daily life
FORM TWO CHEMISTRY EXAM SERIES 126
FORM TWO CHEMISTRY EXAM SERIES 126
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM TWO EXAMINATION – SEPTEMBER 2022
032 CHEMISTRY
TIME: 2:30 HOURS
INSTRUCTIONS
- This paper consists of sections A and B with a total of TEN (10) questions
- Answer all questions in the space provided.
- All writing must be in black or blue ink except for diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Zn= 65, X =32, O=16
SECTION A (15 Marks)
Answer all questions in this section
- For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the bracket provided.
- When burning a fuel produces blue color it means there is
- Adequate supply of oxygen with production of soot
- In adequate supply of oxygen with production of soot
- Adequate supply of oxygen with production of less heat
- Adequate supply of oxygen with production of more heat ( )
- Which of the following is an agricultural chemical products made by the application of chemistry?
- Drugs B. Pesticides C. Clothes D. Cement ( )
- Which of the following is NOT among the composition of air?
- Noble gases B. Hydrogen C. Carbon dioxide D. Nitrogen ( )
- Which one of the following sets of laboratory apparatus are used for measuring volume of liquids
- Conical flask, measuring cylinder and test tube
- Burette, pipette and measuring cylinder ( )
- Volumetric flask, water though and burette
- Gas jar, measuring cylinder and pipette
- The following are the characteristic of ionic compound
- They are easily vaporized
- They are easily dissolved in organic solvent
- Conduct electricity when in solution or molten
- Usually exist as liquid or gas at room temperature
- Which of the following is a symbol of silver ion
- Ag B. S C. Ag+ D. Si ( )
- What is proton, electron and atomic number AI ( )
- 13,13,13 B. 13,11,13 C. 13,12,13 D. 13,13,11
- Which state is involved when drying wet clothes?
- Liquid to solid B. Gas to liquid C. Liquid to gas D. Solid to gas( )
- The components of fire triangle are ;
- Oxygen, fuel and heat C. Oxygen, heat and hydrogen ( )
- Oxygen, nitrogen and heat D. Oxygen, carbon dioxide and fuel
- Which group and period does the element with 14 electrons belong?
- Group II and period 3 C. Group III and period 3 ( )
- Group IV and period 3 D. Group II and period 4
- Match the descriptions in List A with the corresponding in List B by writing the letter of the correct response beside the item number in the answer booklet provided
| LIST A | LIST B |
|
|
Answers
| LIST A | i | ii | iii | iv | v |
| LIST B |
|
|
|
|
|
SECTION B (70 Marks)
Answer all questions in this section
3(a) Fill the blanks
- The techniques used to separate serum from blood samples is called _________________________
- The insoluble substances remain in a filter paper during filtration are termed as ___________________________
- Boiling points of substance reflect the strength of __________________
- The sub atomic particles of an atom are ____________________ and _____________________
- Solar energy is example of _______________________resources
- (b) Suggest the best method of separating the following mixture
- Alcohol and water _______________________________________________________________________________________________________________________________________________________________________________________
- Sodium chloride and water _______________________________________________________________________________________________________________________________________________________________________________________
- Green solution from leaves _______________________________________________________________________________________________________________________________________________________________________________________
- Sand from rice _______________________________________________________________________________________________________________________________________________________________________________________
- Iron fillings and powder calcium carbonate _______________________________________________________________________________________________________________________________________________________________________________________
- (a) Complete the following table
| Class of fire | Materials | Fire extinguisher | Chemical composition of extinguisher |
| CLASS A |
|
| Ordinary tap water pressurized by air |
|
| Flammable liquids | Dry powder extinguisher |
|
| CLASS C |
| Sand bucket
|
|
|
| Metal |
| Sulphuric acid and sodium hydrogen carbonate |
| CLASS E |
|
| Protein and fluoro protein |
(b) With one example explain each of the following
- Alkali earth metals __________________________________________________________________________________________________________________________
- Metalloids __________________________________________________________________________________________________________________________
- Transition metals __________________________________________________________________________________________________________________________
- Why are noble gases stable? __________________________________________________________________________________________________________________________
- Why covalent compound do not conduct electricity __________________________________________________________________________________________________________________________
- (a) What is destructive distillation
__________________________________________________________________________________________________________________________________________
(b) Mention five (5) procedures of preparing charcoal from the method mentioned in
5(a) above
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
(c) Mention four (4) steps of lightning a Bunsen burner
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- ________________________________________________________
- An atom of element X having atomic number 11 combines with an atom of element Z having atomic number 17 to form a compound
- Write the formula of the compound and state the type of bond formed in the compound
- Give four properties of compound in 6(a)
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
- (a) Write the chemical symbols for beryllium, boron, neon, nitrogen and phosphorus
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
(b) You are provided with a compound composed of 40.5% zinc. 19.6% sulphur and
39.9% oxygen. Calculate the molecular and empirical formula. If it’s molecular
mass is 161
- (a) By giving one reason, explain the following facts
- During laboratory preparation of oxygen gas, little manganese dioxide is added to hydrogen peroxide.
__________________________________________________________________________________________________________________________
- Fish can obtain oxygen for respiration although spend their life in water
_________________________________________________________________________________________________________________________
- Oxygen gas can be used for welding activities although it does not burn
__________________________________________________________________________________________________________________________
(b) Which property enables the use of hydrogen gas in
- Fueling __________________________________________________
- Manufacturing of margarine _________________________________
- Give two domestic uses of oxygen gas
- ________________________________
- ________________________________
- (a) Define the following terms
- Water treatment __________________________________________________ ________________________________________________________________
- Water purification _____________________________________________ ________________________________________________________________
(b) List down four (4) economic uses of water
- ____________________________________
- ____________________________________
- ____________________________________
- ____________________________________
(c) What are the importance of water treatment and purification? Give two (2) reasons
__________________________________________________________________________________________________________________________________________
SECTION C (15 Marks)
- Briefly explain five methods used to prevent rusting and give example for each
Page 1 of 6
FORM TWO CHEMISTRY EXAM SERIES 111
FORM TWO CHEMISTRY EXAM SERIES 111
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM TWO-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- The valency of an element with atomic number 10 is;
- 2 B. 3 C. 0 D. 1
- How many protons, neutrons and electrons are there in an atom represented by the symbol
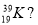
Protons Neutrons Electrons
- 39 19 20
- 19 39 20
- 20 19 20
- 19 20 19
- Separation of a mixture by fraction all distillation is possible if the mixture constituents differ in their;
- Boiling points B. Melting points C. Freezing points D. Vapourizing points
- When element T of Group 1 combines with element X of Group VI, the formula of the compound formed is;
- T2X B. X2T C. TX2 D. XT4
- Saturated solution is one which;
- Contains more solute undissolved at a given temperature
- Has a large amount of solvent at a given temperature
- Contains a little solute at a given temperature
- Will take no more of solute at a given temperature
- The most abundant element on the earth is;
- Carbon B. Iron C. Nitrogen D. Oxygen
- Petrol is an example of;
- Ionic substance C. Flammable substance
- Irritating substance D. Corrosive substance
- Domestic utensils made of iron undergo rusting when exposed to;
A. Air and fire B. Air and oil C. Air and water D. Water and oil
- The chloride iron (Cl-) differs from chlorine atom because chloride ion has;
A. Loss electrons B. Less protons C. More electrons D. More protons
x. Which of the following chemical species have the same number of electrons?
A. Cl, Be, He and O2- B. K+, Ca2+, Cl- and Ar
C. Na+, Mg2+, Al3+ and Li D. O2-, F-, S2- and Cl-
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
SECTION B: 20 MARKS
- You are provided with two lists A and B. Choose a words from list B which matches the statement or phrase in List A and write its letter in the table provided below;
| LIST A | LIST B |
|
|
(b)
SECTION B: 80 MARKS
- (a) What do you understand by the following terms?
- First Aid ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- First Aid Kit ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Name four components which can be found in First Aid Kit;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
(c) Why is it important to provide First Aid to an injured person? Give four reasons;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) For each of the following classes of fire, state the burning material(s);
- Class A fire ……………………………………………………………………………………………
- Class B fire ……………………………………………………………………………………………
- Class C fire ……………………………………………………………………………………………
(b) Fire can be prevented by;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) By giving one example, define the following terms;
- Suspension ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Chromatography ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Briefly explain the following ways of preventing rusting.
- Electroplating ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Galvanizing ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Write the chemical symbols for the following elements;
- Silver ………………………..
- Iron ………………………….
- Aluminium …………………….
- (a) If a mixture of manganese (IV) oxide, MnO2 with potassium chlorate, KClO3 is treated, which of
the two compounds will produce oxygen?
- …………………………………………………………………………………………………
- Why? ………………………………………………………………………………………….
(b) Calculate the oxidation number of the underlined elements;
- NH4+
- SO42-
- CUSO4
- FeCl2
- (a) List down four assumptions of the Dalton’s Atomic Theory;
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
(b) Write a chemical formula for each of the following compounds;
- Water …………………………………………………………..
- Potassium chloride ………………………………………………
- Magnesium oxide ………………………………………………….
- Calcium hydroxide ……………………………………………….
(c) (i) What do you understand by the term “Chemical warning signs”? ……………………………………………………………………………………………………………
(ii) Draw the chemical warning sign that represents;
| HARMFUL OR IRRITANT | TOXIC | FLAMMABLE |
|
|
|
|
- Study the periodic table below;
![]()
![]() I VIII
I VIII
II III IV V VI VII
|
C | A |
D |
|
|
|
|
E |
|
F |
|
|
|
|
G |
H |
|
|
|
J |
|
|
|
|
|
|
Use the letters shown in the periodic table above to indicate;
- Elements with zero valency …………………………
- The lightest atom. …………………………………….
- The alkaline earth match. ……………………………………
- An element with electronic configuration of 2: 8: 1. …………………………..
- Give the names of elements represented by the mentioned letters A, B, C and D. ……………………………………………………………………………………
- Give the name of J as an element. ………………………………………………….
- Write the electronic configuration of J. …………………………………………….
- What type of a bond can be formed when element J combined with element H? ……………………………………………………………………
- What type of a bond can be formed when an element H combines with an element H …………………………………………………………………
- (a) What is air? ………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Mention the composition of air with their percentage composition;
| COMPOSITION | PERCENTAGE (%) COMPOSITION |
|
|
…………………………………….. …………………………………….. …………………………………….. ……………………………………... |
(c) Give four reasons why air is a mixture.
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
iv …………………………………………………………………………………………………
- (a) Give the method used to separate the following mixtures;
- Kerosene and water …………………………………………………………………………………
- Alcohol (ethanol) and water ………………………………………………………………………..
- Iron fillings and sand ……………………………………………………………………………….
- Iodine crystals and table salt ……………………………………………………………………….
(b) Water is a chemical compound? Give four reasons to support this fact.
- ………………………………………………………………………………………………………
- ……………………………………………………………………………………………………….
- ……………………………………………………………………………………………………….
- ………………………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 97
FORM TWO CHEMISTRY EXAM SERIES 97
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM TWO- MARCH/APRIL-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- Write the letter of the best answer in each of the following multiple choice questions. Use the table below.
- What best knowledge can be used by industrialists to manufacture chemicals
- Methods of separating mixtures
- Debate on drug abuse
- Campaigns on elections
- Labeling apparatus for experiments
- How students can protects him against injuries and deaths in a school laboratory?
- To walk very slowly along the corridors of a laboratory
- To study ways of offering first AID
- To observe laboratory rules
- To study uses of products from manufactures
- Which one of the following pair of chemicals is used to swab clean fresh cuts and wounds?
- Methylated spirit and iodine tincture
- Petroleum jelly and copper sulphate
- Milk of magnesia and sodium chloride
- Ethanoic acid and calcium hydroxide
- What is the common use of the following hardware laboratory equipments, a pair of tongs, forceps, clamp and stand, sugar tongs
- To determine mass of the chemicals
- To burn chemicals
- To pick solid chemicals
- To hold objects
- Which is the appropriate warning sign to be pasted on a container that has a chemical which is a source of oxygen
- Flammable
- Explosive
- Oxidizing
- Corrosive
- Which set of apparatus seems to belong in a group of glassware
- Thermometer, crucible and lid, white tile clamp and stand
- Volumetric flask, reagent bottle, beehive shelf, pipe clay triangle
- Separating funnel, burette, pipette, conical flask
- Beakers, cork borer, Bunsen burner, glass rod
- What is the name of the constant temperature of a substance changing its state from liquid to solid?
- Melting point
- Boiling point
- Freezing point
- Sublimation point
- Which process changes a gas directly to solid without the liquid state?
- Condensation
- Sublimation
- Solidification
- Non of the above
- Which of the following process is not a chemical change?
- Soaring of milk
- Burning of paper
- Decomposition of a compound by heating
- Separating mixtures
- Substances which are poor conductors of heat and electricity. What is best idea one can give about the above statement?
- They are metals
- They are non metals
- They are elements
- They are mixtures
- (a) Match the following items by writing the letter against numbers for the best matches. Use the table below
| LIST A | LIST B |
|
|
(b) Fill in the following blanks spaces to make meaningful statement
- …………………………………… is used to separate liquids mixtures of different densities which do not mix. The liquid which comes out first is found at the …………………………………… of the funnel
- A reagent bottle is used to store chemical ……………………………… and the label on the side of the bottle carries the ……………………….of the chemical in the bottle
- Hydrogen gas is a ………………………………… causing substance suck substances are called ……………………………………
- …………………………………… is a small special box which contains items used to offer …………………………………………….
- Detergents are examples of …………………………………pollutants. They interfere with …………………………….balance. B.O.D
SECTION B (80 Marks)
Answer all questions in this section.
- List four (4) chemical properties of oxygen
- Write three (3) common methods of preparations of oxygen in the laboratory
- What is chemical test for oxygen gas?
- List any four (4) uses oxygen gas in our daily life
- Write a word chemical equation to show the decomposition of hydrogen peroxide in the presence of manganese (IV) oxide.
- Why oxygen gas is collected over water?
- Respiration and burning are similar process in some ways and difference process in other ways. Give two differences between them.
10. a) State any four laboratory rules
(b) Consider the diagram below and then answer the questions that follows:
Label parts
- A ………………………………………………………………………………………
- B…………………………………………………………………………………………
- C…………………………………………………………………………………………
- D ………………………………………………………………………………………
- E ……………………………………………………………………………………………..
FORM TWO CHEMISTRY EXAM SERIES 85
FORM TWO CHEMISTRY EXAM SERIES 85
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
ANNUAL EXAMINATION
FORM TWO-2021
CHEMISTRY
032
TIME: 2:30 HOURS November, 2021
![]()
Instructions
1.This paper consists of sections A and B with a total of ten (10) questions.
2.Answer all questions in the spaces provided.
3.All writings must be in blue or black ink except drawings which must be in pencil.
4.All communication devices and calculators are not allowed in the examination room.
5.Write Your Examination Number on the top right of every page.
6.The following atomic masses may be used.
H = 1, C = 12, O = 16, N = 14, S = 32, Zn = 65, Cl = 35.5, Na = 23.
| FOR EXAMINER’S USE ONLY | ||
| QUESTION NUMBER | SCORE | EXAMINER’S INITIALS |
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 | ||
| 10 | ||
| TOTAL | ||
| CHECKER’S INITIALS | ||
SECTION A: (20 Marks)
1.Write down the letter corresponding to the most correct answer in the box provided for each item.
(i) How many protons, neutrons and electrons are there in an atom represented by ![]() ?
?
B.17 18 17
C.18 17 35
D. 17 35 18.
(ii) Which of the following methods is used to separate salt from sea water?
A.Filtration
B.Decantation
C.Decantation
D.Fractional distillation.
(iii)Which of the following warning signs is likely to appear on a gallon containing methylated spirit in the laboratory?
A.Irritant
B.Flammable
C.Oxidant D. Explosion.
(v)Hydrogen peroxide ![]() (iv) X + water. “X” represents _________
(iv) X + water. “X” represents _________
A.Oxygen
B.Hydrogen
C.Nitrogen
D. Carbondioxide.
(vi)If a Bunsen burner produces much soot, which is the correct conclusion?
A.The air hole is closed
B.The burner gas jet is big
C.The air hole is fully opened
D.The gas supply is poor.
(vii)Which of the following is the list of group (II) element?
A.Sodium and magnesium
B.Calcium and carbon
C.Magnesium and calcium
D.Boron and Beryllium.
(viii)Element M of group I combines with element X of group VI, the formula of the compound formed when M combines with X is:
A.MX2
B.MX6
C.X2m
D.M2X.
(ix)The pair of elements which is most likely to form a covalent bond when reacted together is:
A.Carbon and chlorine
B.Aluminium and oxygen
C.Magnesium and oxygen
D.Sodium and chlorine.
(x)One of the following can distinguish hydrogen gas from other gases: A. It burns and supports combustion
B.It is colourless and ordourless C.It gives a smell of a rotten egg
D.It burns with pop sound.
(xi)The following sentences suggest that air is a mixture, except:
A.Its components can be separated physically
B.Its composition varies from one place to another
C.Its properties depends on individual gases
D.Its formation requires absorption or reabsorption of heat.
2.(a) Choose a word(s) from list B which matches the statement or phrases in list A and write its letter in the space provided.
| LIST A | LIST B |
| (i)Factors that can be manipulated to get desired results (ii)A factor that is kept constant (iii)Scientist’s best possible answer (iv)Statement of how the results related to hypothesis (v)First step in scientific method of studying a problem | A.Controlled variable B.Hypothesis C.Observation D.Conclusion E.Independent variable F.Identification of a problem G.Experimentation H.Data analysis |
Answer:
| List A | (i) | (ii) | (iii) | (iv) | (v) |
| List B |
2. (b) Fill in the blanks below with the correct words.
(i)A candle flame is not good for glass ware because _____________________
(ii)A catalyst used for the production of oxygen gas from hydrogen peroxide is _______________________
(iii)A sudden unexpected occurrence that destroy properties and cause injury is called __________________________
(iv)It is used as fuel in rockets due to its lightness _________________________
(v)Blockage of the upper part of airway by food or other objects is called
________________________________
SECTION B: (80 Marks)
Answer all questions in this section
3. (a) What is fuel?
___________________________________________________________________________
_____________________________________________________________________
(b) (i) State four types of fuels used by many Tanzanians:
_______________________________________________________________
_______________________________________________________________
_______________________________________________________________
_______________________________________________________________
@ 1 mark = 04 marks
(ii) Why do you think many Tanzanians prefer to use the mentioned sources in 3b(i) above? Give two best reasons.
_______________________________________________________________
_______________________________________________________________
@ 02 marks = 04 marks
4. You are provided with the following materials from chemistry laboratory:
Apparati: Thistle funnel, delivery tube, beehive self, trough, gas jar, flat bottomed flask with its coke.
Chemicals:Water, zinc granules, dilute hydrochloric acid.
(a)What chemical substance can be prepared using the materials mentioned above?
_____________________________________________________________________
(b)Draw the diagram showing how you could arrange the Apparati above in order to prepare a chemical substance you mentioned in 4(a) above. 05 marks
(c)How can the products mentioned in 4(a) above be collected?____________________________________________________________________
____________________________________________________________________
02 marks
(d) Give at least three (3) uses of the product you mentioned in 4(a) above based on its properties.
(i)______________________________________________________________
(ii)______________________________________________________________
(iii)______________________________________________________________
5. (a) What should you do immediately if:
(i)The piece of broken beaker cuts your finger
_______________________________________________________________
(ii)Chemicals splash on your face
_______________________________________________________________
(iii)Your shirt has caught fire
_______________________________________________________________
(iv)Your fellow student swallows unknown chemical substance thinking that it was water
_______________________________________________________________
@ 02 marks = 08 marks
(b) Why do you think first aid is an important thing? Give two points:
(i)___________________________________________________________
(ii)___________________________________________________________
@ 01 mark = 02 marks
6. (a) (i) A table salt is a common name for the compound with the formula NaCl.
Write the systematic name for the table salt
_______________________________________________________________
_______________________________________________________________
01 mark
(ii) Briefly describe why molecular formula better preferred than empirical formula is_______________________________________________________________ _______________________________________________________________
_______________________________________________________________
02 marks
(b) A compound consists of 27% sodium, 16.5% nitrogen and 56.5% oxygen by mass. If its molecular mass is 85g, find:
(i)Its empirical formula 05 marks
(ii)Molecular formula 02 marks
7. (a) Briefly explain the following terms:
(i)Periodicity
_______________________________________________________________
_______________________________________________________________
(ii)Electronegativity
_______________________________________________________________
_______________________________________________________________
(iii)Ionization energy
_______________________________________________________________
_______________________________________________________________
(iv)Atomic radii/size.
_______________________________________________________________
_______________________________________________________________
@ 01 mark = 04 marks
(b) Why are certain elements in the period table referred to as metalloids? Give two examples of such elements:
Reason: ____________________________________________________________
02 marks
Example:
(i)_____________________________________________________________
(ii)_____________________________________________________________
@ 00 ½ mark
(c) Element X has atomic number of 19 and neutron number 20. Write down its:
(i) Electronic configuration
______________________________________________________________
01 mark
(ii) Mass number 02 marks
_______________________________________________________________
_______________________________________________________________
_______________________________________________________________
8.(a) (i) Differentiate combustion from rusting.
_______________________________________________________________
_______________________________________________________________
(ii) Explain why cars and corrugated iron sheets in coastal areas like Dar es
Salaam, Tanga and Zanzibar get rust more quickly than in other cities of
Tanzania like Mbeya, Mwanza and Arusha? 02 marks
_______________________________________________________________
_______________________________________________________________
(b)Explain how you can put off each of the following types of fire
(i)A fire started by petrol in a container 02 marks
_______________________________________________________________
_______________________________________________________________
(ii)A fire started by a short circuit in an electric wire 02 marks
_______________________________________________________________
(iii)A fire caused by a burning magnesium metal 02 marks
_______________________________________________________________
9.(a) With an example give the meaning of the following terms as applied in Chemistry:
(i)A radical 02 marks
_______________________________________________________________
_______________________________________________________________
(ii)Oxidation state 02 marks_______________________________________________________________ _______________________________________________________________
(b)Find the oxidation numbers of the following underlined elements:
(i) Na 01mark (ii) HNO3 01mark
(iii) H2SO4 01mark (iv) SO32- 01mark
(c) Draw diagrams showing the electron transfer in the formation of MgCl2.
10. (a) Differentiate the following terms:
(i)An element and an atom
__________________________________________________________________
__________________________________________________________________
(ii)A compound and a mixture
__________________________________________________________________
__________________________________________________________________
(b) Melting of ice and burning of papers are two processes that happen almost daily in our life.
(i)Identify the two actions as being physical or chemical change.
Ice melting __________________________________________________
Paper burning ________________________________________________
(ii)Mention two significant things that happen when the paper is burnt:
_______________________________________________________________ _______________________________________________________________
(c) What are the names of elements represented by the following symbols?
(i)K _________________________________________________________
(ii)S _________________________________________________________
(d) Why some elements have their symbols written in one letter while others have two letters? (e.g. N and Na)
FORM TWO CHEMISTRY EXAM SERIES 74
FORM TWO CHEMISTRY EXAM SERIES 74
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM TWO- AUG/SEPT 2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
(i) The elements which are found in group VIII of the periodic table are known as:
- Metals
- Noble gases
- Non - metals
- Right elements
(ii) Isotopes are atoms which have:
- Different mass number
- Different number of electrons
- Different number of protons
- The same number of neutrons
(iii) The elements which are found in group VIII of the periodic table are known as:
- Metals
- Noble gases
- Non - metals
- Right elements
(iv) The process used to separate a mixture of salt and water is:
- Evaporation
- Filtration
- Simple distillation
- Sublimation
(v) Saturated solution is one which:
- Contains more solute undissolved at a given temperature.
- Will take no more of solute at a given temperature.
- Contains a little solute at a given temperature.
- Has a large amount of solvent at given temperature.
(vi) Which of the following electronic configurations are of metals?
- 2:8:8:1 and 2:8:8:7
- 2:8:3 and 2:8
- 2:8:8:1 and 2:8:3
- 2:8:6 and 2:8:8:7
(vii) One isotope of an element has atomic number A and mass number M. How many neutrons are contained in the nucleus of its atom?
- M
- A
- A - M
- M - A
(viii) The total number of protons and neutrons in the nucleus of an atom is called:
- Valency number
- Atomic number
- Molecule number
- Mass number
(ix) Oxidation may be defined as:
- Loss of hydrogen by a substance
- Gain of hydrogen by a substance
- Reaction in which oxygen is lost
- Reaction in which electrons are increased
(x) Moving across a period in the periodic table,
- Electro negativity decreases
- Electro negativity increases
- Metallic property increases
- Electro positivity increases
2. (a)Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| LIST A | LIST B |
|
|
(b) Answer the given questions by writing the correct answer in the blank spaces provided.
- Has both metallic and non metallic properties………………………
- A gas that can undergo sublimation………………………..
- Name given to group seven elements which means salt producer…………………..
- An example of renewable fuel from organic matter…………………..
- Separation of colored substances…………………..
3. Briefly explain five characteristics to be considered when looking for a good fuel.
4.(a) During preparation of Hydrogen gas by the reaction between dilute Hydrochloric acid and Zinc granules, the granules slowly dissolve in acid to form solution X.
(i) Name solution X .........
(ii) Write the chemical formula of X . . . . . .
(b) How can hydrogen gas be tested?![]()
(c) Mention four (4) chemical properties of hydrogen gas.
(d) List three (3) uses of Hydrogen gas.
5.Outline six common apparatus used in the laboratory preparation of oxygen gas using hydrogen peroxide.
(b) Outline four uses of oxygen in everyday life situation.
6.(a) Define the following terms:
(i)Valency
(ii)Oxidation state
(iii) Anion
(iv)Cation
(b) Calculate the oxidation state of the underlined elements in the following radicals:
(i) NH4+
(ii) SO42+
(iii) CLO3-
(c) A compound consists of 40% carbon, 6.67% hydrogen and 53.33% oxygen. If its relative molecular mass is 60, calculate the following:
(i)Empirical formula
(ii)Molecular formula
7.(a) Define the following terms:
(i)Oxidation state ![]()
(ii)An element .......„
(iii)A compound
(iv)Fainting ![]()
(b)Write the chemical formula for each of the following compounds:
(i)Sodium sulphate ![]()
(ii)Sodium chloride .. ![]()
(iii)Calcium nitrate ......... (iv) Calcium oxide . ![]()
8.State two chemical properties of water.
(b) Calculate the molar mass of each of the following compounds:
(ii) NaHC03 (iii) Fe203 ![]()
9.Define the following terms:
(i)Empirical formula .........
(ii)Molecular formula .........
(b)A compound consists of 85.7% carbon and 14.3% hydrogen by mass. If its relative molecular mass is 56, calculate:
(i)Empirical formula.
(ii)Molecular formula.
10.(a) Study the following periodic table and then answer the questions that follow:

(i) Name elements named by the following letters: S, W, X, Z
(ii) Write the electronic configuration for the elements represented by the following letter.
10.(b) Study the experiment diagram below and answer the questions that follow.
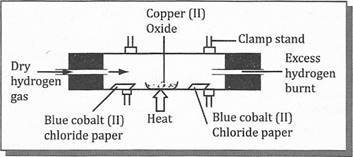
(i)What happens to the copper (Il) oxide during the experiment?
(ii)What happens to the two pieces of cobalt paper? (iii) Write a word equation for the reaction.
FORM TWO CHEMISTRY EXAM SERIES 65
FORM TWO CHEMISTRY EXAM SERIES 65
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRYTERMINALEXAMINATION
FORM TWO-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- The valency of an element with atomic number 10 is;
- 2 B. 3 C. 0 D. 1
- How many protons, neutrons and electrons are there in an atom represented by the symbol
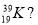
Protons Neutrons Electrons
- 39 19 20
- 19 39 20
- 20 19 20
- 19 20 19
- Separation of a mixture by fraction all distillation is possible if the mixture constituents differ in their;
- Boiling points B. Melting points C. Freezing points D. Vapourizing points
- When element T of Group 1 combines with element X of Group VI, the formula of the compound formed is;
- T2X B. X2T C. TX2 D. XT4
- Saturated solution is one which;
- Contains more solute undissolved at a given temperature
- Has a large amount of solvent at a given temperature
- Contains a little solute at a given temperature
- Will take no more of solute at a given temperature
- The most abundant element on the earth is;
- Carbon B. Iron C. Nitrogen D. Oxygen
- Petrol is an example of;
- Ionic substance C. Flammable substance
- Irritating substance D. Corrosive substance
- Domestic utensils made of iron undergo rusting when exposed to;
A. Air and fire B. Air and oil C. Air and water D. Water and oil
- The chloride iron (Cl-) differs from chlorine atom because chloride ion has;
A. Loss electrons B. Less protons C. More electrons D. More protons
x. Which of the following chemical species have the same number of electrons?
A. Cl, Be, He and O2- B. K+, Ca2+, Cl- and Ar
C. Na+, Mg2+, Al3+ and Li D. O2-, F-, S2- and Cl-
2. (a) You are provided with two lists A and B. Choose a words from list B which matches the statement or phrase in List A and write its letter in the table provided below;
| LIST A | LIST B |
|
|
SECTION B: 80 MARKS
- (a) What do you understand by the following terms?
- First Aid ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- First Aid Kit ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Name four components which can be found in First Aid Kit;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
(c) Why is it important to provide First Aid to an injured person? Give four reasons;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) For each of the following classes of fire, state the burning material(s);
- Class A fire ……………………………………………………………………………………………
- Class B fire ……………………………………………………………………………………………
- Class C fire ……………………………………………………………………………………………
(b) Fire can be prevented by;
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- …………………………………………………………………………………………………………
- (a) By giving one example, define the following terms;
- Suspension ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Chromatography ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(b) Briefly explain the following ways of preventing rusting.
- Electroplating ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
- Galvanizing ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Write the chemical symbols for the following elements;
- Silver ………………………..
- Iron ………………………….
- Aluminium …………………….
- (a) If a mixture of manganese (IV) oxide, MnO2 with potassium chlorate, KClO3 is treated, which of
the two compounds will produce oxygen?
- …………………………………………………………………………………………………
- Why? ………………………………………………………………………………………….
(b) Calculate the oxidation number of the underlined elements;
- NH4+
- SO42-
- CUSO4
- FeCl2
- (a) List down four assumptions of the Dalton’s Atomic Theory;
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
(b) Write a chemical formula for each of the following compounds;
- Water …………………………………………………………..
- Potassium chloride ………………………………………………
- Magnesium oxide ………………………………………………….
- Calcium hydroxide ……………………………………………….
(c) (i) What do you understand by the term “Chemical warning signs”? ……………………………………………………………………………………………………………
(ii) Draw the chemical warning sign that represents;
| HARMFUL OR IRRITANT | TOXIC | FLAMMABLE |
- Study the periodic table below;
![]()
![]() I VIII
I VIII
II III IV V VI VII
| C | A | D | E | ||||
| F | G | H | |||||
| J |
Use the letters shown in the periodic table above to indicate;
- Elements with zero valency …………………………
- The lightest atom. …………………………………….
- The alkaline earth match. ……………………………………
- An element with electronic configuration of 2: 8: 1. …………………………..
- Give the names of elements represented by the mentioned letters A, B, C and D. ……………………………………………………………………………………
- Give the name of J as an element. ………………………………………………….
- Write the electronic configuration of J. …………………………………………….
- What type of a bond can be formed when element J combined with element H? ……………………………………………………………………
- What type of a bond can be formed when an element H combines with an element H …………………………………………………………………
- (a) What is air? ………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Mention the composition of air with their percentage composition;
| COMPOSITION | PERCENTAGE (%) COMPOSITION |
| …………………………………….. …………………………………….. …………………………………….. ……………………………………... |
(c) Give four reasons why air is a mixture.
- ……………………………………………………………………………………………………
- …………………………………………………………………………………………………
- …………………………………………………………………………………………………
iv …………………………………………………………………………………………………
- (a) Give the method used to separate the following mixtures;
- Kerosene and water …………………………………………………………………………………
- Alcohol (ethanol) and water ………………………………………………………………………..
- Iron fillings and sand ……………………………………………………………………………….
- Iodine crystals and table salt ……………………………………………………………………….
(b) Water is a chemical compound? Give four reasons to support this fact.
- ………………………………………………………………………………………………………
- ……………………………………………………………………………………………………….
- ……………………………………………………………………………………………………….
- ………………………………………………………………………………………………………
FORM TWO CHEMISTRY EXAM SERIES 57
FORM TWO CHEMISTRY EXAM SERIES 57
Candidate’s Examination Number ________________________
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY MID TERM EXAMINATION-MARCH
FORM TWO-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(x), choose the correct answer from the alternatives given.
- Isotopes are atoms of the same element that have different
- Atomic number
- Electronic arrangement
- Mass number
- Protons
- The process by which water is converted into water vapour or steam is called
- Condensation
- Evaporation
- Precipitation
- Transpiration
- In the Bunsen burner a sooty flame is most likely to be formed when the
- Air holes are fully closed
- Air holes are opened
- Flame is noisy
- Flame is smaller
- The best method to separate a mixture of iodine and iron is
- Decantation
- Evaporation to dryness
- Fractional to dryness
- Sublimation
- In scientific study, the tentative explanation of the observed chemical phenomenon can be proved by
- Data analysis
- Experimentation
- Hypothesis
- Observation
- The choice of the source of heat depends on the_____
- Colour of the flame
- Quantity of heat produced
- Substance to be burned or boiled
- Type and shape of the flame
- When oxygen combines with metal they:-
- Form metallic oxides
- Form precipitation
- Form rust
- Sublime
- When dilute hydrochloric acid react with metal the product is______
- Hydrogen chloride gas
- Hydrogen gas
- Chloride gas
- Hydrogen peroxide
- Hydrogen react with sulphur to yield_______
- Hydrogen sulphide
- hydrogen sulphate
- Sulphur dioxide
- Sulphur trioxide
- The product smell obtained from the combustion of sulphur in air is_____
- Pleasant
- Toxic
- pungent
- Harmful
| i | ii | Iii | iv | v | Vi | vii | viii | ix | x |
2. (a) You are provided with two list A and list B choose a statement which match the word in list A and write appropriate word in the space provided
| LIST A | LIST B |
|
|
| I | Ii | Iii | Iv | v |
(b) Fill the blanks for each of the following
- _____________________is the smallest particle of an element that has all the chemical properties of that element.
- _______________________is a negative charged particle with a mass of approximately equal to
 .
. - _________________________is the process of making water clean and safe for human consumption
- __________________________is the process where y aluminium sulphate and impurities in water clump together and sink to the bottom of container
- Water treatment is ________________________________________________________________________________________________________________________________________________
SECTION B (80 Marks)
3. (a) Use a diagram to explain the preparation of hydrogen using dilute hydrochloric acid and zinc granules
(b) Briefly explain four uses of hydrogen
(i)______________________________________________________________________________________________________________________________________________ (ii)_____________________________________________________________________________________________________________________________________________ (iii)_____________________________________________________________________________________________________________________________________________ (iv)_____________________________________________________________________________________________________________________________________________
4. (a) List four Dalton’s atomic theories
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
(b) State four modifications of Dalton’s atomic theories
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
(c) Mention two shortcomings of Dalton’s atomic theory
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
5. (a) Write down six physical properties of water
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- Give four reasons why water is important in our daily lives and industry.
- ______________________________________________________________________________________________________________________________
- ______________________________________________________________________________________________________________________________
- ______________________________________________________________________________________________________________________________
- ______________________________________________________________________________________________________________________________
6. (a) Give the meaning of each of the following sub atomic particles
- Proton
____________________________________________________________________________________________________________________________________
- Electron
____________________________________________________________________________________________________________________________________
- Neutron
____________________________________________________________________________________________________________________________________
(b) Differentiate between physical and chemical change of matter (five differences)
| Physical change | Chemical change |
| i. | |
| ii. | |
| iii. | |
| iv. | |
| v. |
(c) (i) Write the equation for the preparation of oxygen by heating potassium chlorate
(ii) How oxygen is tested in the laboratory
________________________________________________________________________________________________________________________________________________
7. (a) With chemical equation, give the product of combustion in oxygen of
- A non metal ____________________________________________________________________________________________________________________________________
- A metal , each of your own choice ____________________________________________________________________________________________________________________________________
(b) (i) Define catalyst ________________________________________________________________________________________________________________________________________________
(ii) In experiment of preparation of hydrogen, why copper (II) sulphate is added into the solution of zinc granules and dilute sulphuric acid? ________________________________________________________________________________________________________________________________________________
- Why were the first few bubbles of gas not collected? ____________________________________________________________________________________________________________________________________
- Write the word equation for the reaction during preparation of hydrogen gas by zinc granules __________________________________________________________________
- Suggest two ways how hydrogen is collected during its preparation ____________________________________________________________________________________________________________________________________
- Suggest one problem with collecting hydrogen by upward delivery, remember hydrogen is colourless ____________________________________________________________________________________________________________________________________
8. (a) Explain four methods of preventing rusting of iron (i)______________________________________________________________________ (ii)_____________________________________________________________________ (iii)_____________________________________________________________________ (iv)_____________________________________________________________________
(b) In the following diagram of interconverting of matter name the processes from a to f
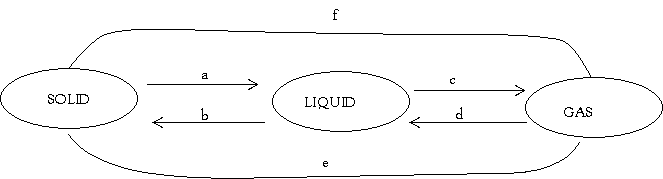
- _________________________________
- _________________________________
- _________________________________
- _________________________________
- _________________________________
- _________________________________
9. (a) Study the diagram below and answer the questions that follow
- What process is taking place? ____________________________________________________________________
- Name the parts labeled A to D
- ______________________________________
- ______________________________________
- ______________________________________
- ______________________________________
(b) For each of the following classes of fire state the burning material and give its suitable fire extinguisher
| Class | Burning material | Fire extinguisher |
| Class A | ||
| Class B | ||
| Class C | ||
| Class D | ||
| Class E |
10. (a) Draw the following warning signs as used in the laboratory, for each give two examples
| Name | Diagram | Examples |
| Toxic | ||
| Flammable | ||
| Oxidant | ||
| Radioactive |
(b) Define each of the following terms
- Warning signs ____________________________________________________________________________________________________________________________________
- Laboratory ____________________________________________________________________________________________________________________________________
FORM TWO CHEMISTRY EXAM SERIES 47
FORM TWO CHEMISTRY EXAM SERIES 47
THE PRESIDENTS OFFICE MINISTRY OF LOCAL GOVERNMENT AND REGIONAL ADMINISTRATION
FORM II CHEMISTRY MIDTERM MARCH-2021
1. For each of the items (i)-(x) choose the correct answer from among the given alternatives and write its letter in the box provided.
- Which of the following does not constitute a fire triangle?
- Oxygen
- Fuel
- Combustible material
- Heat.
- Why is oxygen regarded as one of the most important part of air?
- It supports combustion
- It is a diatomic gas
- It forms the largest part of air
- It has the largest density.
- Which is not a property of oxygen gas?
- Colorless
- Odorless
- Burns
- Denser than air.
- A simple proof that chemical reaction takes place in our bodies is that;
- We occasionally fall sick
- Doctors tell us in hospitals
- The food or drink we take is different from the waste product produced
- We cook food
- A chemist will require the following skills except?
- Experimentation
- Observation
- Problem identification
- Surgery
- Burning back in Bunsen burner occur when
- Air hole is closed
- Bunsen burner is overheated
- Gas supply is high
- Air hole is fully open
- The most common source of heat in laboratory is
- Kerosine stove
- Liquefied petroleum
- Spirit lamb
- Bunsen burner
- A gas was passed into test tube containing lime water. The lime water turned milky. The gas was mostly likely to be;
- Sulphur dioxide
- Carbon monoxide
- Carbon dioxide
- Nitrogen dioxide.
- In which class of fire will you put kerosine?
- Class A
- Class B
- Class C
- Class E
- What do you call an instrument used to hold and heat chemicals?
- Measuring cylinder
- Gas jar
- Test tube
- Funnel
2. Match the correct answer in list A with alternative given in list B by writing the answer of the correct answer in the spaces provided.
| LIST A | LIST B |
|
|
3. Complete the following statements with the correct answers
- A substance that can burn is said to be . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- A gas that occupies the largest part of atmosphere . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Another name for anodizing as method of preventing rusting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Chemical name of rust . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Liquid obtained after distillation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- (a) Write six methods that can be used to prevent rusting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Mention the conditions required for rusting
5. (a) Which property of hydrogen make it to be used in the following ways
- Oxyhydrogen flame
- Weather balloon
- Manufacture of margarine
(b) Which property of oxygen makes to be used as follows
- Respiration
- Cutting metals and welding
- Removal of impurities
- (a) Define the following terms
- Laboratory
- First aid
- First aid kit
- Matter
(b) Mention five laboratory rules
7. (a) Name the chemicals mostly used in laboratory to prepare oxygen.
(b) State the way you can test for presence of oxygen
(c) Explain four chemical properties of oxygen.
8. (a) By giving an example in each case, define the following terms
- A chemical symbol
- An element
- A compound
- A suspension
(b) Write the names of the elements presented by the following symbols
- Au
- Ag
- Hg
- P
- Sn
9. (a) Mention five accidents that can happen in laboratory?
(b) State five ways we can do to minimize accidents in laboratory
10. (a) What is a flame?
(b) Mention two types of flames
(c) Explain why Bunsen burner is the best flame to use in laboratory
(d) Give differences between luminous and non-luminous flame
FORM TWO CHEMISTRY EXAM SERIES 38
FORM TWO CHEMISTRY EXAM SERIES 38
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
AUGUST-SEPTEMBER EXAMINATION SERIES
CHEMISTRY FORM-2
2020
TIME: 2:30 HRS
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) Chemistry is defined as:
- The scientific study of matter, compounds and chemical reactions
- The scientific study of compounds, mixtures and organic substances
- The scientific study of composition, structure and properties of matter
- The study of relation between human being, medicine and pollution.
(ii) A non-luminous flame is the most applicable flame for heating purposes because:
- It is very noisy
- It has no soot
- It is very hot
- It has no colour
(iii) Matter is defined as anything that has:
- Volume and occupies space
- Mass and occupies space
- Mass and occupies density
- Density and space
(iv) Which of the following process is used in preventing rust of an iron?
- Water
- Boiling
- Salting
- Galvanization
(v) Water is a universal solvent because:
- It is available everywhere
- It boils at 1000C
- It dissolves most of the solutes
- It dissolves all crystals in compounds
(vi) Which among the following are the two processes involved during distillation?
- Evaporation and sublimation
- Evaporation and crystallization
- Evaporation and condensation
- Evaporation and decantation
(vii) Which of the following set of nuclide notation represents isotopes?
- 18 X 16 X 19 x
- 18 X 18 X X
- 16 x 18 x 1890x
- 16 X 1789x,
(viii) The chemical used to test the presence of water in a substance is:
A. Cobalt Il oxide B. Cobalt 111 oxide
C. Cobalt chloride D. Copper Il chloride
![]() (ix) When a burning fuel produces blue colour it means there is:
(ix) When a burning fuel produces blue colour it means there is:
A. adequate supply of oxygen with production of soot
B. inadequate supply of oxygen with production of more heat.
![]() C. inadequate supply of oxygen with production of soot.
C. inadequate supply of oxygen with production of soot.
D. adequate supply of oxygen with production of more heat.
![]() (x) Which of the following is the best apparatus for measuring accurately a fixed volume of a given solution?
(x) Which of the following is the best apparatus for measuring accurately a fixed volume of a given solution?
A. Measuring cylinder B. Beaker
C. Pipette D. Burette
2. Match each item in List A with a correct response in List B by writing its letter bellow the number of the corresponding item in the table provided.
| LIST A | LIST B |
| A. Metalloids B. Non-metals C. Periodicity D. T r a n s i t i o n elements E. Electro negativity F. Alkali metals G. Halogens H. Periodic law I. Alkali earth metals J. Rare non metals K. Period L. Noble gases M. Periodic table N. Group |
SECTION B. 80 MARKS
3.(a) Define the following terms:
(i)Oxidation state ![]()
(ii)An element .......„
(iii)A compound
(iv)Fainting
(b)Write the chemical formula for each of the following compounds:
(i)Sodium sulphate ![]()
(ii)Sodium chloride .. ![]()
(iii)Calcium nitrate ......... (iv) Calcium oxide .
4.Gas X can be prepared in the laboratory by the decomposition of hydrogen peroxide.
(a)Identify gas X .. ![]()
(b)State three physical properties of Gas X
![]() Mention three chemical properties of gas X. (d) State three uses of gas X.
Mention three chemical properties of gas X. (d) State three uses of gas X.
5.Write the name of each of the following compounds:
(i)(NH4)2C03
(ii)CaCl2
(iii) NaSO4
(iv) KC103
(b)Give three differences between the following:
(i) ![]() Electrovalent compounds and covalent compounds. (ii) Solutions and suspensions.
Electrovalent compounds and covalent compounds. (ii) Solutions and suspensions.
6.(a) Define the following terms:
(i)Covalent bond
(ii)Electrovalent bond
(b) A compound consists of 82.8% carbon and 17.2% hydrogen by mass. The vapour density of the compound is 29. Calculate:
(i)Empirical formula.
(ii)Molecular formula.
7.(a) Define the following terms as applied in Chemistry:
(i)Flame ![]()
(ii)Bunsen burner
(iii)Laboratory
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
8.(a) Write a word equation for each of the following reactions:
(i)Calcium burns in Oxygen .........
(ii)Sodium reacts with water ... ... ...
(b) What do you understand by the following terms?
(i) Water treatment
(ii) Water purification
(c) Mention six uses of water in economic activities.
9.(a) Mention four physical properties of water.
(b) What will happen when:
(i) a burning splint of wood is introduced into a gas jar containing oxygen gas
(ii) oxygen gas reacts with metals .
(iii) hydrogen gas reacts with oxygen gas .........
(c) List four uses of hydrogen in our daily life.
10. What do you understand by the term "valency"?
(b) Calculate the oxidation number of the underlined elements: (i) NaOH
(i) CO3
(iii) Na3P04
(iv) NO2
(v) S02
(c) State the importance of changes of state in daily life.
FORM TWO CHEMISTRY EXAM SERIES 31
FORM TWO CHEMISTRY EXAM SERIES 31
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- BACK TO SCHOOL EXAMINATION-JUNE
FORM TWO
Time 2:30 Hours JUNE 2020
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) What is the best way of keeping a clean test tube after use?
- Keeping it in water
- Keeping it on a test tube holder
- Keeping it in a basin for test tubes
- Keeping it on a test tube rack.
(ii) What happens when substance A reacts with substance B to form a new substance C?
- Substance A and B are said to have formed a solution
- Substance A and B are said to have undergone a physical change.
- Substance A and B are said to have undergone a chemical change.
- Substance A and B are said to have undergone a dissolution.
(iii) Why Non – luminous flame is the most applicable flame for heating purposes?
- It is very nosy
- It has no soot.
- It is very hot
- It has air holes open
(iv) How many zones are there in a non-luminous flame?
- Four zones
- Two zones
- Three zones
- Five zones
(v) When a small amount of sugar is dissolved in a glass of water the mixture formed is:
- heterogeneous.
- immiscible.
- suspension.
- homogenous.
(vi) A change from gaseous state to solid state without passing through a liquid state is called:
- Deposition
- Sublimation
- Condensation
- Solidification
(vii) Which among the following are the two processes involved during distillation?
- Evaporation and sublimation
- Evaporation and crystallization
- Evaporation and condensation
- Evaporation and decantation
(viii) Technicians prefer to use blue flame in welding because:
- It is bright and non-sooty
- It is light and non-sooty
- It is very hot and large
- It is very hot and non-sooty
(ix) Which of the following is a sequential method of separating mixture of salt and sand?
- Evaporation, filtration and decantation
- Decantation, evaporation and filtration
- Sedimentation, evaporation and filtration
- Decantation, filtration and evaporation
(x) The factors that affect the problem being investigated are referred to as:
- Dependent factors
- Variables
- Independent factors
- Conditions
2. Match each item in List A with a correct response in List B by writing its letter bellow the number of the corresponding item in the table provided.
| LIST A | LIST B |
| (i) Method of recovery of both solute and solvent from a liquid. (ii) Method of separating two miscible liquids which their boiling points are close together. (iii) Method of separating two immiscible liquids. (iv) Method of separating two solids by heating in a way that one changes its state directly to gas. (v) Method of separating coloured components using a moving solvent on materials that absorb such solvent.
|
|
(b) Fill in the blank spaces by using the appropriate terms.
- Smallest particles that makes up matter ……………………………….
- The substance obtained after filtration is called……………………..
- The factors that make distillation possible is difference in………….
- Directly change from solid to gas………………………………….
- The temperature at which a liquid changes into gas…………………
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) State one use of each of the items (i) – (x) in administering First Aid.
| S/N | Item | Use |
| (i) | Soap |
|
| (ii) | Bandage |
|
| (iii) | Sterile gauze |
|
| (iv) | Iodine tincture |
|
| (v) | Petroleum jelly |
|
b. (i) Write the name and chemical formula of two important chemical substances used in the laboratory preparation of oxygen gas.
(ii) Mention four physical properties of oxygen gas.
4. (a) What are the five steps used in lighting a Bunsen burner?
(b) Classify fuels based on their physical state and for each class give two examples.
5.(a) Study the experiment diagram below and answer the questions that follow.
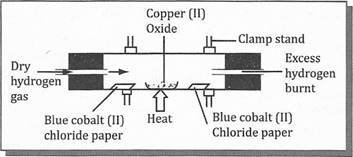
(i)What happens to the copper (Il) oxide during the experiment?
(ii)What happens to the two pieces of cobalt paper? (iii) Write a word equation for the reaction.
(b) Mention four chemical properties of hydrogen gas.
6.(a) Define the following terms as applied in Chemistry:
(i)Flame ![]()
(ii)Bunsen burner
(iii)Laboratory
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
7.(a) Define the following terms:
(i)Chemistry
(ii)Element
(iii)Catalyst
(b) Give three differences between the following:
(i) Compound and mixture
(ii) Suspension and solution
8. (a) Write a word equation for each of the following reactions:
(i)Calcium burns in Oxygen .........
(ii)Sodium reacts with water ... ... ...
(b) What do you understand by the following terms?
(i) Water treatment
(ii) Water purification
(c) Mention six uses of water in economic activities.
9. State the law of conservation of energy.
(b) Give two ways in which energy can be transformed from one form to another.
(c) List down two sources of heat in the laboratory.
10.(a) Define the term "empirical formula".
(b) An organic compound contains 26.7% carbon, 2.2% hydrogen and
71.1% oxygen. If its relative molecular mass is 90, determine its:
(i)Empirical formula
(ii)Molecular formula
(c ) State the modern atomic theory ideas.
FORM TWO CHEMISTRY EXAM SERIES 24
FORM TWO CHEMISTRY EXAM SERIES 24
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- TERMINAL EXAMINATION-MAY
FORM TWO
TIME: 2HRS 2020
NAME:_____________________________________CLASS:___________
INSTRUCTIONS
- Do all questions from sections A, B and C.
- All working must be shown clearly and neatness
- The following atomic masses may be used;
H = 1, C = 12, O = 16, N = 14, Ca = 40, K = 39.
- All writing must be done in black or blue ink EXCEPT for the diagrams which must be in pencil.
SECTION A: 10 MARKS
1. Write down the letter corresponding to the correct answer for each item (i) – (x).
(i) The valency of an element with atomic number 10 is;
- 2
- 3
- 0
- 1
(ii) How many protons, neutrons and electrons are there in an atom represented by the symbol ![]()
Protons Neutrons Electrons
- 39 19 20
- 19 39 20
- 20 19 20
- 19 20 19
(iii) Separation of a mixture by fraction all distillation is possible if the mixture constituents differ in their;
- Boiling points
- Melting points
- Freezing points
- Vapourizing points
(iv) When element T of Group 1 combines with element X of Group VI, the formula of the compound formed is;
- T2X
- X2T
- TX2
- XT4
(v) Saturated solution is one which;
- Contains more solute undissolved at a given temperature
- Has a large amount of solvent at a given temperature
- Contains a little solute at a given temperature
- Will take no more of solute at a given temperature
(vi) The most abundant element on the earth is;
- Carbon
- Iron
- Nitrogen
- Oxygen
(vii) Petrol is an example of;
- Ionic substance
- Irritating substance
- Flammable substance
- Corrosive substance
(viii) Domestic utensils made of iron undergo rusting when exposed to;
- Air and fire
- Air and oil
- Air and water
- Water and oil
(ix) The chloride iron (Cl-) differs from chlorine atom because chloride ion has;
- Loss electrons
- Less protons
- More electrons
- More protons
(x) Which of the following chemical species have the same number of electrons?
- Cl, Be, He and O2-
- K+, Ca2+, Cl- and Ar
- Na+, Mg2+, Al3+ and Li
- O2-, F-, S2- and Cl-
SECTION B: 20 MARKS
2. You are provided with two lists A and B. Choose a words from list B which matches the statement or phrase in List A and write its letter in the table provided below;
| LIST A | LIST B |
|
|
SECTION C: 70 MARKS
- (a) What do you understand by the following terms?
- First Aid …
- First Aid Kit
(b) Name four components which can be found in First Aid Kit;
(c) Why is it important to provide First Aid to an injured person? Give four reasons;
4. (a) For each of the following classes of fire, state the burning material(s);
- Class A fire
- Class B fire
- Class C fire
(b) Fire can be prevented by;
5. (a) By giving one example, define the following terms;
- Suspension
- Chromatography
(b) Briefly explain the following ways of preventing rusting.
- Electroplating
- Galvanizing
(c) Write the chemical symbols for the following elements;
- Silver ………………………..
- Iron ………………………….
- Aluminium …………………….
6. (a) If a mixture of manganese (IV) oxide, MnO2 with potassium chlorate, KClO3 is treated, which of the two compounds will produce oxygen?
- ……………………………………
- Why? ……………………………………
(b) Calculate the oxidation number of the underlined elements;
- NH4+
- SO42-
- CUSO4
- FeCl2
7. (a) List down four assumptions of the Dalton’s Atomic Theory;
(b) Write a chemical formula for each of the following compounds;
- Water ……………………
- Potassium chloride ……… …
- Magnesium oxide …………
- Calcium hydroxide ………… .
(c) (i) What do you understand by the term “Chemical warning signs”?
(ii) Draw the chemical warning sign that represents;
| HARMFUL OR IRRITANT | TOXIC | FLAMMABLE |
|
|
|
|
8. Study the periodic table below;
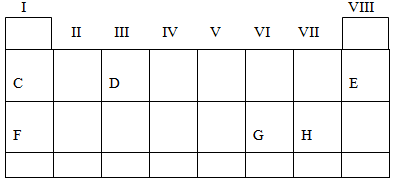
Use the letters shown in the periodic table above to indicate;
- Elements with zero valency ………………
- The lightest atom. ……………………… .
- The alkaline earth match. ………………
- An element with electronic configuration of 2: 8: 1. …………………………..
- Give the names of elements represented by the mentioned letters A, B, C and D. …………………
- Give the name of J as an element. ………………………
- Write the electronic configuration of J. …………………… ……………….
- What type of a bond can be formed when element J combined with element H? ………………………… …
- What type of a bond can be formed when an element H combines with an element H ………………………
9. (a) What is air?
(b) Mention the composition of air with their percentage composition;
| COMPOSITION | PERCENTAGE (%) COMPOSITION |
|
|
(c) Give four reasons why air is a mixture.
10. (a) Give the method used to separate the following mixtures;
- Kerosene and water …………………
- Alcohol (ethanol) and water …………………
- Iron fillings and sand …………………………
- Iodine crystals and table salt …………… …….
(b) What is a chemical compound? Give four reasons to support this fact.
FORM TWO CHEMISTRY EXAM SERIES 15
FORM TWO CHEMISTRY EXAM SERIES 15
THE PRESIDENT’S OFFICE
THE MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
MID TERM EXAMINATIONS-MARCH 2020
032 CHEMISTRY
Duration: 2:30 Hours
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) All domestic utensils made of iron undergo rusting when exposed to:
- Air and fire
- Air and oil
- Air and water
- Water and oil
(ii) When a small amount of common salt is dissolved in glass of water the mixture formed is:
- Heterogeneous
- Homogeneous
- Immiscible
- Suspension
(iii) A chemist should acquire all of the following skills except:
- Experimentation
- Observation
- Problem identification
- Surgery
(iv) An important property of oxygen which distinguishes it from other gases is that it:
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor supports combustion
- Supports combustion but does not burn.
(v) The process of chlorination in water treatment aims at:
- Forming suspension
- Killing micro – organisms
- Making syrup
- Removing bad odour
(vi) Class E fire can best be extinguished by using:
- Carbon dioxide
- Fire blanket
- Sand
- Water
(vii) The following is a set of apparatuses which are used for heating:
- Crucible, test tube, evaporating dish
- Evaporating dish, tongs, crucible
- Test tube, evaporating dish, tongs
- Tongs, crucible, test tube.
(viii) Which of the following methods can be used to get oil from cotton seeds?
- Decantation
- Distillation
- Grinding and distillation
- Grinding followed by squeezing.
(ix) The substance that can be used to extinguish fire are:
- Carbon dioxide and sand
- Carbon dioxide and sugar
- Nitrogen and sand
- Nitrogen and water
(x) When sugar is dissolved in water, a uniform mixture is formed. The resulting mixture is called a:
- Solute
- Solution
- Solvent
- Suspension
2.(a) Match each item in List A with a response in List B by writing its letter below the number of the corresponding item in the table provided.
| List A | List B |
|
|
(b) Fill in the blank spaces by using the appropriate terms
- In an atom, the effect of the charged nucleons is balanced by the charge of……………..
- Serum is separated from blood samples by employing a technique called………………..
- Boiling points of substances reflect the strength of……………….
- Grinding chalk into a powder involves changing the state of …………………….
- The insoluble substances formed during filtration are collectively termed as ……………….
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) Mariam was preparing food foe her family using hot oil in a frying pan. Accidentally the pan tripped over and a huge fire spread over her kitchen floor.
- Mention two extinguishers which would be appropriate for putting out the fire.
- Which fire extinguisher would be dangerous to use when trying to put out the fire in (a) above? Give reason.
(b) Mention three conditions for a fire to start
(c) (i) What is combustion?
(ii) Give three areas where combustion is applied.
4. (a) In an experiment, two iron nails A and B were used whereby painting was applied on nail A. The two nails were placed in a moist environment and after one month the weight of each nail was determined. Which of the two nails would be heavier? Give reason.
(b) State the method which will be used to protect each of the following from rusting:
- Covering iron sheets with a layer of most reactive metals
- Bicycle chain
5. (a) List down four careers that are a result of studying chemistry.
(b) The following are possible causes of accidents which can occur in the Chemistry laboratory. State how you can avoid them.
- Poisonous chemicals left in an unlocked cupboard
- A student picking up a bottle containing concentrated H2SO4 acid by the neck
- Concentrated acids stored in the upper most shelf of cupboard.
6. (a) Mention four physical properties of water
(b) What will happen when?
- A burning splint of wood is introduced into a gas jar containing oxygen gas
- Oxygen gas reacts with metals
- Hydrogen gas reacts with oxygen gas
(c) List four uses of hydrogen in our daily life.
7. (a) Define the following terms as applied in Chemistry.
- Flame
- Bunsen burner
- Laboratory
(b) List four properties of each of the following:
- A luminous flame
- A non – luminous flame
(c) Give the use of each of the following components which are found in the First Aid Kit.
- Plaster
- A pair of scissors
- Cotton wool
- Gloves.
8. (a) Define the following terms;
- Chemistry
- Element
- Catalyst
(b) Give three differences between the following:
- Compound and mixture
- Suspension and solution.
9. Gas “L” has the following properties: it is highly flammable, readily combines with other elements, readily reacts with other chemical substances and is a strong reducing agent.
- Name the gas “L”
- What is the method used to collect gas”L” in the laboratory? Give a reason.
- Give four uses of gas “L”
10. (a) Draw a diagram to show laboratory preparation of oxygen using hydrogen peroxide. In the diagram, label all the compounds and elements involved in the preparation.
(b) Briefly explain how you would distinguish ordinary air from pure oxygen.
(c) List two chemical properties of oxygen gas
FORM TWO CHEMISTRY EXAM SERIES 3
FORM TWO CHEMISTRY EXAM SERIES 3
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256