THE UNITED REPUBLIC OF TANZANIA THE PRESIDENT’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
KIGOMA DISTRICT COUNCIL

FORM TWO DISTRICT MOCK EXAMINATION
CHEMISTRY
Wednesday, 21st May, 2025.
Instructions:
- This paper consists of sections A, B and C with a total of ten (10) questions
- Answer all questions in all sections
- Cellular phones and any unauthorized materials are not allowed in the examination room
- Write your EXAMINATION NUMBER on every page of your booklet(s)
- Use a blue or black pen and pencil should be used for diagrams.
- The following atomic masses may be used H = 1, N = 14, O = 16, Na = 23
SECTION A (15 Marks)
Answer all questions in this section
1. For each of the items (i) - (x), choose the correct answer from the given alternatives and write its letter in the box provided.
(i) In scientific study, the tentative explanation for the observed chemical phenomenon can be proved by:
- Data analysis
- Experimentation
- Hypothesis
- Observation
(ii) The fixed volume of distilled water in the laboratory can be measured by using:
- Beaker
- Burette
- Pipette
- Small measuring cylinder
(iii) Which of the following sets of process represent uses of oxygen gas?
- Welding, ice melting, magnetization
- Mountaineering, sublimation, freezing
- Glass cutting, desiccation welding
- Diving, welding, mountaineering.
(iv) Which of the following fire extinguishing items will you see to help your friend whose shirt caught fire during an experiment in the laboratory?
- Foam
- Carbon dioxide
- Water
- Fire blanket
(v) What type of fire occur in the vapor air mixture over the surface of flammable liquids
- Class A
- Class B
- Class C
- Class D
(vi) A Form I students wants to prepare a solution in the laboratory. He has to have
- Water and petrol
- A solute and colloid
- A solute and a solvent
- An insoluble salt and water
(vii) Which common feature is associated with elements of the same group?
- Equal number of protons
- Equal number of electrons
- Equal number of valence electrons
- Equal number of shells.
(viii) An element in the periodic table with atomic number 18 belong to which of the following?
- Group I and period I
- Group O and period III .
- Group III and period III
- Group V and period IV
(ix) Water is a universal solvent because:
- It is available everywhere
- It boils at 1000
- It dissolves most of the solvent
- It dissolves all crystals
(x) The amount of air entering the Bunsen burner can be controlled by adjusting the opening of the
- collar
- Jet
- Base
- Barrel
2. Match the items in list A with the responses in list B by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
| (i) Means that a substance is dangerous and can cause death within a short time (ii) Means that a substance is dangerous and can affect our health for long time (iii) Means that the substance emits harmful radiations that penetrate human body and cause damage. (iv) Means that the substance can speed up the rate of burning. (v) Means that the substance can catch fire easily |
|
SECTION B (70 Marks)
Answer all questions in this section
3. (a) Mr. Otuu in the rural areas was interested to know how the knowledge of chemistry is necessary in agricultural activities. As a chemist, educate Mr. Otuu on the application of chemistry in agriculture by giving four points
(b) A teacher from a certain school bought a small box which had a sign of Red Cross. Some of form one students they did not have a knowledge about that box by using knowledge that you have learnt from chemistry answer the following questions.
(i) What is the name given to that bo
(ii) Briefly explain the functions of four possible items that can be found in a box
4. When her child was suffering from cold, Mrs. Justin was prescribed with a medical syrup wrote “shake well before use” on its sideward.
(a) (i)What does this mean?
(ii) Give the chemical name of this syrup
(b) (b) i. Define the chemical name you provided above.
(ii) Name three properties of the chemical name provided above.
5. (a) Vaines was instructed by his father to buy a bag of 1kg of sugar, while returning home the bag busted and all the sugar mixed with sand
(i) With the concept of separation of mixtures explain how Vaines could obtain sugar
(ii) Name all methods of separation of mixtures involved
(b) How can you separate mixtures in each of the following:
- Oil from seeds
- Muddy from water
- Green colour from chlorophy II
- Iron fillings from sand
- Alcohol from water
- Sand and rice
6. When zinc granules and dilute sulphuric acid are reacted together a gas M is produced. The gas is collected by down ward displacement of water. Use this information to answer the questions below:
(a) i. Name the gas M produced.
ii. How is the gas tested?
iii. Why is the gas collected by downward displacement of water?
(c) (i) Write the balanced reaction of zinc granules with dilute sulphuric acid
(ii) What happens if gas M reacts with Copper (II) oxide?
(iii) Write the balance reaction when gas M combines with oxygen.
7. (a) Changes of matter is very important in our daily life. (Give four reasons)
(b) Identify the following processes whether is physical or chemical change
- Jewellery tarnishes ________________________________________________
- Clay is moulded into a new shape ____________________________________
- Food craps are turned into compost in compost pit _______________________
- The juice in the bottles freezes _______________________________________
- Burning a candle __________________________________________________
- A match is lit____________________________________________________
8. (a) State what will be observed when the following simple experiment are performed
(i) A piece of paper (White) is placed into luminous flame
________________________________________________________________ ________________________________________________________________
(ii) A glowing splint is lowered into a jar full of oxygen
________________________________________________________________ ________________________________________________________________
(iii) When Iron bar is exposed to air for five nights
________________________________________________________________ ________________________________________________________________
(iv) When white copper (ii) sulphate paper is inserted in pure water
________________________________________________________________ ________________________________________________________________
(b) Account the following events
(i) Water is used to put off class E fire
________________________________________________________________ ________________________________________________________________
(ii) Group VIII elements do not form compound
________________________________________________________ ________________________________________________________________
9. (a) A compound consists of 27% sodium, 16.5% nitrogen and 56.5% oxygen by mass. Calculate the simplest formula of the compound .
(b) During the practical session, Asha found some bottles in the laboratory containing different chemical compounds with different chemical formulas, unfortunately she failed to write the IUPAC names of these chemical compounds. As form two student help her by writing the correct IUPAC names of these chemical compounds found by Asha on bottles in the laboratory.
(i) KCl _____________________________________________________________
(ii) CaCl2 ___________________________________________________________
(iii) AlCl3 ____________________________________________________________
(iv) SF6 ____________________________________________________________
SECTION C (15 Marks)
10. (a) Casimiro is a form two student at Nakwa Secondary School, he always prepares a breakfast before going to school, but he realized that much of his time is used in the kitchen preparing tea, which advice would you give to him upon the fuel he used.
(b) For him to have a good fuel what are other consideration he should make before buying another fuel. Give four
(c) If the fuel used by Casimiro is not environmental friend, what comment will you make to him so that to conserve environment and their natural heritages. Give two comments
FORM TWO CHEMISTRY EXAM SERIES 117
FORM TWO CHEMISTRY EXAM SERIES 117
THE UNITED REPUBLIC OF TANZANIA PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM TWO MOCK II EXAMINATION CODE
032 CHEMISTRY
TIME: 2:30 Hours May, 2024
INSTRUCTIONS
- This paper consists of ten (10) questions in two sections A and B each question carries a total of 10 marks.
- Answer all questions in all sections
- All writing must be in BLUE/BLACK ink EXCEPT drawings which must be drawn by using a pencil.
- Write your index number in every page of your answer sheet.
- All answers must be written only in the spaces provided in each question and not otherwise.
SECTION A.
1. For each of the items (i)-(x) choose the correct answers from the given alternatives and write its letter in the table below: (10 marks)
(i) Which among the following lists contains the items in transport that are produced as a result of application of chemistry?
- Dyes, tyres and fuels.
- Coolant, fuel and lubricants
- Paints, lubricants and pesticides
- Lubricants, tyres, and drugs
(ii) Which of the following groups of substances represents flammable liquids?
- Petrol, pesticides and hydrogen
- Petrol, sulphuric acid and methylated spirit
- Methylated spirit, petrol and kerosene
- Kerosene, diesel and hot water.
(iii) The chemistry teacher of form one asked his students to make a class presentation about the sequential steps of separating a mixture of salt and sand. Which of the following list is in a correct series.
- Sedimentation, evaporation and filtration.
- Decantation, filtration and evaporation.
- Evaporation, filtration and decantation.
- Decantation, evaporation and filtration.
(iv) Which of the following compounds is binary.
- Magnesium hydroxide, Mg(OH)2
- Magnesium phosphate, Mg3(PO4)2
- Magnesium nitride, Mg3N2
- Magnesium carbonate, MgCO3
(v) Which term describes a rapid chemical reaction that releases energy in the form of light and heat?
- Ignition
- Combustion
- Fire
- Heating
(vi) Any problem in the scientific research is affected by atleast two factors commonly referred to as variables. Which variable change its values when the values of other variables change?
- Constant variable.
- Independent variable
- Dependent variable
- Manipulated variable.
(vii) The following is one of the reason why water is termed as the universal solvent;
- Water dissolve many solutes than any other solvent
- Water boils at 100 oC and freezes at 0 oC
- Water has high surface tension.
- Water is colourless, odorless and tasteless liquid
(viii) Which of the following is the chemical substance used to test the presence of water vapour in air by changing the blue anhydrous to pink hydrated compound;
- Copper II sulphate
- Calcium chloride
- Cobalt II chloride
- Potassium permanganate
(ix) Which of the following is the reverse of deposition process.
- Melting
- Freezing
- Alloying
- Sublimation
(x) Which of the following process cannot be grouped into physical change.
- Cutting of iron into pieces
- Grinding of charcoal into powder
- Heating of copper metal
- Burning of zinc metal TABLE
| Question | (i) | (ii) | (iii) | (iv) | (v) | (vi) | (vii) | (viii) | (ix) | (x) |
| Answers |
2. Match the materials in List A with the correct method of preventing it from rusting in List B by writing the letter of the correct answer in the table below the item number in the table provided. (10 marks)
| List A | List B |
|
|
Answer
| i | ii | iii | iv | v |
SECTION B
3. (a) Define the term fuel. (2 marks)
(b) Write down any three uses of fuels in our daily life. (3 marks)
3. One of the form two student in school X decided to boil water by using fire wood charcoal fuel. She had 6kg mass of charcoal fuel, and 2litres of pure water with 30oC, after boiling water the charcoal and all the remained ashes were re-measured to be 2kg. Calculate the heat value of charcoal fuel used above. (Given that specific heat capacity of pure water, Cw = 4.2KjKg-K-, Density of pure water=1000KgM-3 (5 marks)
4. (a) Define the term rust. (2 marks)
(b) Write down the chemical formula of rust. (2 marks)
(c) Briefly explain why iron in salt water rust faster than in fresh water? (3 marks)
(d) List down three disadvantages of rusting process in our daily life. (3 marks)
5. (a) Give out the reason why oxygen gas is normally collected by the method called downward displacement of water. (2 marks)
(b) Briefly explain how you would test the presence of the following gases in air.
- Oxygen . (2 marks)
- Carbon dioxide (2 marks)
(c) With four reasons explain why air is not termed as a compound. (4 marks)
6. (a) Define the term experimentation as used in scientific procedures. (2 marks)
(b) The father of one of the form two student was complaint about the failure of his daughter in science subjects after receiving his daughter’s annual academic report. He decided to ask his daughter what lead her to fail in those subjects, she said that because she is not good in mathematics while most of these subjects involves the applications of mathematics subject, she added that the language used is well understood and is the same to all secondary examinations.
Use the above information to respond in the following questions.
- Write down the problem identified in above information. (1 marks)
- What down the hypothesis formulated in above information. (1 marks)
- Name the following variable found in the above information.
- Dependent variable . (1 marks)
- Independent variable .. (1 marks)
- Controlled variable . (1 marks)
(c) Write down any three applications of scientific procedures in our daily life. (3 marks).
7. (a) Define the term element. (2 marks)
(b) Write down three properties of electrovalent bond. (3 marks)
(c) Write down the Latin name and chemical symbol of the following element. (6 marks).
| Element | Latin name | Chemical symbol |
| Tungsten | ||
| Silver | ||
| Mercury |
8. (a) Define the term suspension as used in chemistry. (2 marks)
(b) List down any two general components of each of the following. (4 marks)
i. Solution = . . . . . . . . . . . . . . and . . . . . . . . . . . . . . . . . . .
ii. Aerosol = . . . . . . . . . . . . . . . And . . . . . . . . . . . . . . . . .
(c) Write down one method of separating mixture used to obtain each of the following substances from its mixture; (4 marks)
- Cooking oil from groundnuts .
- Chlorophyll or green pigment from plant leaf
- Oxygen gas from air
- Sulphur from mixture of Sulphur and sand .
9. (a) Define the following terms;
- Atom (2 marks)
- Isotopes (2 marks)
(b) Fill the following table of the components of atom or sub atomic particles. (3 marks)
| Sub atomic particle | Charge | Location in the atom |
| Proton | . . . . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . |
| . . . . . . . . . . . . . . . . . . . . . . | Zero | . . . . . . . . . . . . . . . . . . . . |
| . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . . . . | Orbit (shell) |
(c) You have given an atom Y with the atomic number of 11, use the atomic number given to respond on the following. (3 marks)
- Write down the proton number of atom Y above.
- Calculate the atomic mass or mass number of atom Y above.
- Calculate neutron number of atom Y above.
10. One of the form one student decided to separate the mixture of cooking oil and water which are mixed accidentally, use this information to respond on the questions below;
- What is the apparatus/equipment which can be used to separate the above mixture? . (2 marks)
- What is the name of the method used to separate this mixture? . (2 marks)
- By using a well labelled diagram explain how those two components can be separated by this student.
Explanation
...................................................................................................................................................... ......................................................................................................................................................
...................................................................................................................................................... ......................................................................................................................................................
...................................................................................................................................................... (3 marks)
FORM TWO CHEMISTRY EXAM SERIES 100
FORM TWO CHEMISTRY EXAM SERIES 100
PRESIDENT’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT-RALG
RUFIJI DISTRICT COUNCIL
DISTRICT FORM TWO COMPETENCE EXAMINATIONS – 2024
032 CHEMISTRY
TIME: 2:30 HRS Monday 25th March, 2024 AM
INSTRUCTIONS
- This paper consist of sections A ,B and C with total of ten (10) questions
- Answer all questions in the space provided
- Section A carries fifteen (15) marks, section B seventy (70) marks and section c carries fifteen (15) Marks
- All writing must being blue or black ink except drawing which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in assessment room
- Write your Index number on every page
SECTION A (15 Marks)
Answer all questions in this section
1. For each of items (i) – (x), choose the correct answer from the given alternatives and write its letter in the box provided.
(i). The welders prefer to use non-luminous flame for the work simply because:
- It is available
- It is easy to transport
- Produce very hot flame
- Can be made by kerosene.
(ii). Identify the name given to burns caused by hot liquids or vapour.
- Injuries
- Scars
- Scalds
- Wounds
(iii). A person at home can apply chemistry in:
- weighing cassava and maize flour
- weeding flowers in a garden
- cooking food in a kitchen
- dusting the floor in a house.
(iv). Factors in an experiment that can be manipulated to get desired results are called;
- Controlled variables
- Manipulated variable
- Dependent variables
- Independent variables.
(v). Which of the following are the two process involved during distillation?
- Evaporation and sublimation
- Evaporation and condensation
- Decantation and evaporation
- Evaporation and crystallization
(vi). Which of the following substances occupies the shape of the container?
- Solid
- Particle
- Liquid
- Crystal.
(vii). Why hydrogen is not a constituent of air?
- Because of being highly flammable
- Because of being a reducing agent
- Because of being very light
- Because of being very reactive/
(viii). How can one prevent rusting in fragile instrument like camera?
- By using silica gel
- using ethanol
- By galvanization
- By using oil;
(ix). The most common industrial method of preparing oxygen;
- Electrolysis of copper sulphate
- Decomposition of hydrogen peroxide
- Electrolysis of bases
- Fractional distillation of liquefied air.
(x). .. is an apparatus used to measure a fixed volume of liquids in the laboratory?
- Test tube
- Beaker
- Pipette
- Measuring cylinder
2. Match each item in list A with correct response in list B by writing the letter of the correct response below the corresponding item number in the table provided
| List A | List B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. (a) Mr. Victor from rural area was interested to know how the knowledge of chemistry is necessary in agriculture activities. As a chemist, educate Mr. Victor on the application of chemistry in agriculture by giving four points.
(b). A teacher from certain school bought a small box which had a sign of Red Cross. Some of form one students they did not have a knowledge about that box. By using knowledge that you have learnt from chemistry subject answer the following questions:
- What is name given to that box?
- Briefly explain the functions of four possible items that can be found in a box.
4. .(a) Which three heat sources can be used to boil some water in the laboratory instead of a Bunsen burner?
(b). Explain why a flame produced by candle is not suitable for cooking. Give two reasons
(b) Arrange the following steps of lighting the Bunsen burner in a correct sequence using letter A to F.
- Turn the collar to let air in through the air hole until you get a non-luminous flame.
- Quickly Ping a flame at the top of the barrel
- Connect the Bunsen burner by rubber tube to the gas source
- Put off the flame of the burner after finish heating by turning the gas tap off.
- Close the air holes.
- Turn the gas tap on to let in sufficient gas.
5. (a) Form one students were arguing if water is a compound or mixture. They went to their Pothers in form two to seek for the correct explanation. What could be the possible explanation of what water is? Water is Because
(b) Someone was instructed by her father to buy a bag of 1kg of sugar while returning home the bag busted and all the sugar mixed with a sand.
- With the concept of separation of mixtures, explain how someone could obtain sugar from sand.
- Name all the methods of separation of mixture involved.
6. Mr. Mkemia is a scientist from Ujiji village; his job is to help the government of Ujiji village to find scientific solutions to the problems that occur in their village because he knows the systematic steps of solving problems scientifically.
- Name the six main steps in correct order that Mr. Mkemia uses to find the scientific solution.
- . what name is given to the steps mentioned in part (a) above?
- Give three applications of named technique in (b) above
7. The laboratory technician planned to conduct an experiment for the preparation of gas M. He decided to use a pieces of zinc metal and dilute hydrochloric acid.
- Identify gas M
- Mention six apparatus that he can use to prepare the gas M.
- Write the word equation for the laboratory preparation of gas M.
- Describe the properties of gas M which relates with its uses. Give two points.
8. (a) Explain the following terms;
- Physical change
- Chemical change
(b) Student of form two performing two simple experiments concerning changes of matter on two substances, A and B in the laboratory. In experiment number 1, student changes substance “A” from solid to liquid and in experiment number 2, student changes substance “B” by burning it to form ashes.
(i) Provide one example of each substance between A and B
(ii) What are the four differences between the changes of matter occurred in substance “A” and the changes of matter that occurred in substance “B”
9. (a) A student was preparing food for her family using hot oil on frying pan. Accidentally, the pan tipped over and a huge fire spread on the kitchen floor.
- Which fire extinguishers would be suitable for putting off the fire?
- Which fire extinguisher would not be suitable for putting off the fire? Explain
(b) Giving a reason, state whether the rust will form or not in each of the situation (i)- (vi)
- Iron bar is dipped into boiling water
- Painted iron is dipped into un-boiled water
- Iron bar is dipped in un-boiled water
- Oiled iron bar is left outside the room over night
- Aluminium wire is dipped in un-boiled water
- A dry iron bar is wrapped with cotton wool
SECTION C (15 Marks)
This section consists only one (1) question
10. . (a)
- Draw a clearly labeled diagram showing the laboratory preparation of oxygen without the application of heat.
- Write the word equation and molecular equation of preparation of oxygen gas from 10. (a)(i)above.
- Name the catalyst used.
(b) What is the method used to collect prepared oxygen gas from 10 (a)(i) above? Give two reasons to support your answer.
(c)
- Why first bubbles should be allowed to escape when collecting oxygen?
- Mention three (3) uses of oxygen gas.
FORM TWO CHEMISTRY EXAM SERIES 93
FORM TWO CHEMISTRY EXAM SERIES 93
CHRISTIAN SOCIAL SERVICES COMMISSION- (CSSC)
WESTERN ZONE

FORM TWO JOINT EXAMINATION
CODE: 032 CHEMISTRY
TIME2:30HRS Date: 30th August, 2023
INSTRUCTIONS:
- This paper consists of section A, B, and C with a total of ten (10) questions.
- Answer all questions in the spaces provided
- Section A carries (15) marks, section B carries (70) marks and section C carries seventy (15) marks.
- All communication devices and any unauthorized materials are not allowed in the examination room.
- Write your examination number on every page
- All writings should be in blue/black ink, all diagrams should be drawn in pencil.
- The following atomic masses may be used.
- H=1, N=14, O=16, Na=23
| QUESTION NO | SCORE | ASSESSOR’S INITIAL |
| 01 | ||
| 02 | ||
| 03 | ||
| 04 | ||
| 05 | ||
| 06 | ||
| 07 | ||
| 08 | ||
| 09 | ||
| 10 | ||
| TOTAL | ||
| CHECKER’S INITIAL | ||
SECTION A: (15 marks)
1. For each items i-x, choose the correct answer from among the given alternatives and write its letter in the space provided.
i. the best method of separating a mixture of sodium chloride and ammonium chloride is:
- Sublimation
- Layer separation
- Decantation
- Chromatography
ii. The formula below represents some chemical substances. Which formula represents hydrated copper (ii) sulphate?
- Cu2 S2 O4
- CuSo2. 5H2O
- CuSO4
- CuSO4. 5H2O
iv. Mwl. Adasa from St. Francis exavier secondary school during making the general cleanliness at her home, she found a bottle of coca cola written the term “composition” the bottle was left by her daughter Divine on the table.. What does the term mean in chemistry?
- Break down of matter
- Waste disposal
- Suspension
- Building up of matter
v. Joel is a meteorologist at Mwanza weather station. He wants to use weather balloons so as to record information on the elements of weather.
Which gas would you advise him to use for filling weather balloons?
- Hydrogen
- Nitrogen gas
- Oxygen gas
- Noble gas
vi. In Haramba’s office, there are many rust utensils made up with element called iron. These utensils can undergo rusting if they are exposed to:
- Air and fire
- Air and water
- Air and palm oil
- Air and soil
vi. Using the abbreviation xyJ and J is an element, x is a mass number and y is atomic number. Choose the list that contains isotopes of the same element.
- 1J2, 3J6, 4J8
- 1J1, 2J1, 3J1
- 1J1, 1J2, 1J3
- 2J2, 4J4, 6J6
vii. An element from Alkali metals reacts with carbonate. What is the probable chemical formula of that Alkali carbonate?
- ACO3
- A2 (CO3)2
- A3CO
- A2CO3
viii. Form one students saw a sheet of paper in the chemical laboratory written as “sulphuric acid is a strong acid”. In warning signs sulphuric acid is …
- Very corrosive chemical
- Highly flammable
- Strong oxidizing agent
- Strong metal
ix. Gabriel gets burnt accidentally during cooking rice at home. he would be given one of the following as first aid:
- Iodine solution
- Gentian violet
- Clinical thermometer
- Soap
x. If you want the Bunsen burner flame to have the same colour as that of a candle, then you must:
- have the air hole fully opened
- have raised the burner
- have the air hole fully closed
- turn off the gas supply
Answers:
| Question | i | ii | iii | iv | v | vi | vii | viii | ix | x |
| Answer |
2. Match the items in list A with response in list B by writing the letter of the correct response in the table provide below.
| LIST A | LIST B |
|
|
Answers
| List A | i | ii | iii | iv | v |
| List B |
SECTION B: (70 marks)
Answer all questions in this section
3. By giving one reason, explain the following facts.
i. During laboratory preparation of oxygen gas little manganese dioxide is added to hydrogen peroxide.
Reason:__________________________________________________________________
__________________________________________________________________
ii. Fish can obtain oxygen for respiration although they spend their lives in water. Reason:__________________________________________________________________
__________________________________________________________________
iii. Oxygen gas can be used for welding activities although it does not burn.
Reason:__________________________________________________________________
__________________________________________________________________
iv. Nyanda gets confusion on how hydrogen gas properties relates with its uses. Assist Nyanda on how the properties of hydrogen can relate with its uses.
| PROPERTY | USES |
| i. | |
| ii. |
4. (a) The structure of the atom is successive improvement of various models advanced by different scientists. John Dalton was the first scientist to suggest on the structure of atom. However, his model failed to explain some observations.
State any four (4) Modifications made on Dalton’s atomic theory.
i. __________________________________________________________________
ii. __________________________________________________________________
iii. __________________________________________________________________
iv. __________________________________________________________________
(b) If the relative atomic mass of element P is 16.2. But P has X% of y8P and 90% 168P. Calculate the value of x and y.
5. Niyonkulu is an expert environmental conservation. He was invited by WEO of Janda village to educate people about the environmental effects of using charcoal as the source of fuel but he did not attend the meeting. As a from two student use that vacancy to educate Janda villager’s. (Three points)
(b) Identify three materials produced from destructive distillation of coal.
(c) A mass of 20.0 g of kerosene was burnt in air. The heat produced was used to heat 2.5 litres of water. Given that the heat value of kerosene is 43, 640 kJ/Kg. By how much the temperature of water could have changed?
Use: The specific heat capacity of water=4.18 kJKg-1K-1 Density of water1000Kg/m3
6. (a) With one example, give the meaning of the following the following terms as applied in chemistry.
i. Radical
__________________________________________________________________
__________________________________________________________________
ii. Oxidation state
__________________________________________________________________ __________________________________________________________________
(b) Find the oxidation number of the following underlined elements.
i. Al
ii. HNO3
iii. Cr2 O72-
iv. Al2(SO4)3
v. S2O32-
7. Mixing of different substances involves chemical and physical changes. state whether the following is physical or chemical change
i. A match is lit _________________________________________
ii. Grinding a piece of chalk ___________________________________________
iii. Rice is cooked _________________________________________
iv. Drying of paper __________________________________________________
v. Iron sheet rust ___________________________________________________
(b) Grace went into the laboratory and found five bottles with different mixtures. The bottles were labeled A, B, C, D and E which contains Alcohol and water, iodine and sand, cooking oil and water, water and salt, maize grain and sand respectively. Which method will she use to separate mixtures on those bottles?
Bottle A- ___________________________________________________________
Bottle B- ___________________________________________________________
Bottle C-_____________________________________________________________
Bottle D-_______________________________________________________________ Bottle E-________________________________________________________________
8. (a) Differentiate between empirical; formula and molecular formula.
Empirical formula
________________________________________________________________________
________________________________________________________________________
Molecular formula
________________________________________________________________________
________________________________________________________________________
(b) An organic compound contains 72.8% carbon, 7.2% hydrogen and the percentage composition rest was for oxygen. If it’s relative molecular mass is 164.
Determine its;
i. Empirical formula
ii. Molecular formula
9. Fill in the following by giving any two products that are made by the application of chemistry in each of the field shown.
| FIELD | PRODUCTS | |
| i. | Medicine | i…………………………. ii………………………… |
| ii. | Transport | i……………………………… ii………………………………. |
| iii. | Agriculture | i………………………………… ii…………………………………… |
| iv. | Food and beverage industries | i………………………………………. ii………………………………………… |
| v. | Textile industry | i…………………………………………. ii…………………………………………… |
(b) State the uses of the following laboratory apparatus
i. Safety goggles ____________________________________________________
ii. Spatula __________________________________________________________
iii. Tongs ____________________________________________________________
iv. Reagent bottle ____________________________________________________
v. Plastic wash bottle ________________________________________________
SECTION C: (15 MARKS)
10. (a) Construct the diagram to show the electronic structure in each of the following compounds
i. Oxygen gas (O2)
ii. Ammonia (NH3)
iii. Table salt
(b) What type of bond exists in the compound in part (a)?
i. Oxygen gas _______________________________________________________
ii. Ammonia ____________________________________________________________
iii. Table salt ______________________________________________________________
(c) Give out three differences on the type of bond exists in the compound (a) i and (iii)
FORM TWO CHEMISTRY EXAM SERIES 74
FORM TWO CHEMISTRY EXAM SERIES 74
THE UNITED REPUBLIC OF TANZANIA PRESIDENT'S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
MOROGORO REGION
FORM TWO REGIONAL ASSESSMENT
CODE: 032 CHEMISTRY -2023
TIME: 2.30 HOURS YEAR: 2023
INSTRUCTIONS
- This paper consists of section A, B, and C with a total of Ten (10) questions.
- Answer all questions in all sections.
- Answer all questions in the spaces provided.
- All writings must be in black or blue ink except diagrams which must be in pencil.

- Cellular phones and any unauthorized materials are not allowed in the examination room.
- The following constants can be used: atomic masses:
- H=l, 0=16, C=12 Na=23, S=32, Specific heat capacity of water 4.18kJkg -1 k-1
SECTION A (15 MARKS)
Answer all question in this section
1. For each of the item (i -x), Choose the most correct answer from among the given alternatives and write its letter in the space provided below.
i) Which of the following is unlikely to produce soot?
- Complete combustion
- incomplete combustion
- limited supply of air burning
- luminous flame
ii) The mixture of cooking oil and water can be termed as
- Suspension
- Emulsion
- Solution
- Saturation
iii) Experimentation in chemistry is used to test.
- Observation.
- Hypotheses.
- Data.
- Problem.
iv). Hydrogen has three isotopes 1H (99.99%), 2H (0.01) and 3H (rare). Its relative atomic mass is?
- 1.10
- 2.01
- 3.10
- 1.001
v) State of matter depend on ![]()
- Tensile strength
- Property of atoms
- Arrangement of particles
- Temperature change
vi) The process of coating iron or s eel with zinc is known as
- Zinc painting.
- Alloying.
- Tin plating.
- Galvanization
vii) A class of fire that cannot be extinguished by water due to different in densities
- Class C
- Class D
- Class E
- Class B
viii) Element with atomic 2 is likely to have similar properties to element with atomic number.
- 18
- 9
- 12
- 7
ix) The gas that turn lime water milky
- Sulphur dioxide
- Nitrogen dioxide
- Carbon dioxide
- Carbon monoxide
x) If K and Y are isotopes of the same element, then K and Y have the same.
- Mass number.
- Atomic weight
- Atomic Number
- Number of neutrons
2. Match the items in List A with the correct response in List B by writing the letter of the most correct answer beside the item number in space provided below.
| LIST A | LIST B |
|
|
SECTION B (70 MARKS)
Answer all questions in this section
3. a) Hydrogen gas is prepared in the laboratory in different ways. The most common method is by the action of dilute acids on metals
i. Draw a neat well labeled diagram for the laboratory preparation of hydrogen gas
ii. Write a balanced chemical equation for this laboratory preparation of hydrogen gas
b) Relating to its property on each explain two uses of hydrogen gas.
b) Given that element 13G reacts with element Using electronic configuration write the formula of the compound formed (G and X are not the actual symbols of the elements).
c) Briefly explain any two importance of changes of states of matter.
5. a) Identify types of change involved in each of the following. i.e. state whether physical or chemical change,
i) Respiration ii) Sublimation iii) Combustion iv) Distillation
b)Write the chemical symbols of the following elements
i) Potassium ii) Sodium iii) Mercury iv) Gold
c) Write the most suitable method of separating the following mixture.
i) Air
ii) Kerosene and water
iij) Iodine and sand
iv) Syrup
6. A compound consists of 27.3% sodium, 1.2% hydrogen, 14.3% carbon a nd oxygen. Its relative atomic mass is 84
i) Calculate its empirical formula
a. Use the answer in 6(i) to find its molecular formula
ii) State the name of the compound
7. a) Briefly explain how periodicity contributed in the preparation of the modern periodic table.
b) Although elements in the periodic table are arranged in such a way that elements with similar properties are placed in the same group. There some trends across the period and down the group. Giving reason(s) for each, briefly explain the trends of electronegativity and electro positivity
i) electro-positivity
ii) electro-negativity
7. (a) Dalton's ideas contributed a lot about the explanation and the atom but does not describe the structure of the atom. State four assumptions of Dalton atomic theory.
b) Assume Boron has two stable isotopes 10B (39.78%) and 11B. Calculate the average atomic mass of Boron.
8. a) Elements 40W has 22 neutrons (the letter is not the actual symbols of the element). State the element's:
i) Atomic number
ii) Number of protons
iii) Electronic configuration
iv) Name of element W
b) Element G is in group 7 period 3.
i) Write the atomic number of G
ii) Write the nuclide notation of two isotopes of G
SECTION C (15 MARKS)
9. a) At Grace's village livestock keeping especially cows is the main economic activity. Most of the villagers use fire wood as their main source of fuel. Educate them on the dangers they are likely to face if they continue using fire wood (four points) and advise them the alternative source of fuel to use.
b) The heat value of kerosene is 43400kJkg-1. Calculate the mass of kerosene that is required to rise the temperature of 20kg of water from 293k to 333k.
FORM TWO CHEMISTRY EXAM SERIES 57
FORM TWO CHEMISTRY EXAM SERIES 57
 | PRESIDENT’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT MVOMERO DISTRICT COUNCIL |  |
FORM TWO MOCK EXAMINATION
032 CHEMISTRY
TIME: 2:30 Hours MAY 2023
Instructions
- This paper consist of sections A, B, and C with total of ten questions
- Answer all question in this paper
- All writing must be in blue or black ink except drawing which must be in pencil
- Write your examination number at the top right corner of every page
- The following atomic masses may be used H=1, N=14, O=16, S=32, Ca= 40
SECTION A (marks 15)
1. For each of the items number i-x choose the correct answer from the given alternatives and write its letter in the box provided
i) Form two students discovered that it is impossible to light fire in a vacuum due to the absence of certain gas. What gas do you think is missing?
- Nitrogen
- Carbon dioxide
- Oxygen
- Hydrogen
- Sulphur dioxide is missing
ii A simple proof that some chemical reactions take place in our bodied is that
- We eat a balanced diet
- Doctor tell us so in the hospital
- We occasionally fall sick
- The food and drinks we take are quite different from the waste product from our bodies
- There is no proof
iii) Why oxygen as one of the components of air is unique?
- It forms largest part of the air
- It is diatomic gas
- It has the largest density
- It supports combustion
- It does not support combustion
iv) What change of state is involved when drying wet clothes
- Gas to solid
- Liquid to gas
- Gas to liquid
- Solid to gas
- Liquid to solid
v) Oxygen gas can be produced at a large scale by
- Fractional distillation of liquefied air
- Condensation of air
- Evaporation of liquefied air
- Condensation of liquefied air
- Liquefaction of steam
vi)Which is the suitable alternative heat sources to be used inabsence of Bunsen burner?
- Kerosene stove and torch
- Torch and spirit burner
- Kerosene, stove, and spirit burner
- Fire wood and torch
- Torch and candle
vii) In water purification using commercial filter hasthe sequence
- Gravel, sand, charcoal, cloth and beaker
- Gravel, sand, charcoal, beaker and cloth
- Sand, gravel, charcoal, gravel, beaker and cloth
- Sand, charcoal, gravel, beaker and cloth
- Sand, beaker cloth,charcoal and gravel
viii)Sodium is abbreviated as Na but both letters are exclusive in the word sodium, thisis because
- The Latinized as naterite
- Comes from the word natural
- The Latin name of sodium is Natrium
- It is derived from the two letters of its English name
- It is derivedfrom English name
ix) Ethanol and water mixture can be separated using
- Layer separation
- Simple distillation
- Evaporation
- Filtration
- Fractional distillation
x)fire triangle is the component required for a fire to start This is
- Heat, fuel and energy
- Heat, fuel and air
- Heat, water and energy
- Heat, ironand light
- Heat, light and energy
2.Match in list A with the responses in List B by writing the letter of the correct response beside the item number in the answer sheet
| LIST A | LIST B |
|
|
SECTION B (70 MARKS)
3 (a) Everybody should be familiar with the instruments and chemicals found in the first aid kit and how to use them.Give any two instruments and two chemicals found in the first aid kit and briefly explain their uses.
(b) Water is one of the basic components in the first aid kit, yetit’s not a must to be found in a kit. Explain
(c) Give two examples of apparatuses that are made up of:
(i) Porcelain /ceramic materials
(ii) Plastic materials
(iii) Glass materials
(iv) Iron materials
4 (a) briefly explain each of the following phenomena
- A ship in sea water, rust very fast compared to a ship in fresh water
- A glowing splint of wood is introduced into a gas jar containing oxygen gas
- Oxygen gas reacts with non- metals
- Hydrogen gas reacts with oxygen gas
- A form two students dipped a clean iron rod into a cold distilled water in a test tube and left it for 2 days, explain observation after two days.
- State what will happens to the iron rod after 2 days?
- Outline three things which cause the process in (b) (i) above
- How could these students prevent the process above despite of the conditions stated. Give two methods.
5 (a) Anisha cuts on her fingers when she was preparing vegetable for lunch, immediately Vasterhelped her before she sends her to Annawema, a doctor.
(i)What was done by Vaster to Anisha?
(ii) Is the help given by Vaster to Anisha important or nothing? State four reasons to support your answer
(b) Explain briefly the following terms
(i) Melting
(ii) Freezing
(c) What are the three importance of changing states of matter from one state to another.
6 (a) Classify the following processes into physical and chemical change.
- Boiling of water to form vapour
- Decaying of teeth
- Rusting of iron
- Magnetization of iron.
- Souring of milk
- Grinding of chalk
- Melting of ice.
(b) Why some processes in (a) above are classified as physical change? Give three points.
7. (a) What do you understand by the word fire?
(b)Albertina burnt a piece of wood and the results were energy and light.
(i) Name the chemical reaction involved.
(ii) What is the name given to a piece of wood in this reaction?
(iii) With one example write three areas where chemical reactions in (b )(i) above is applied.
8. The laboratory technician planned to conduct an experiment for the preparation of gas M. He decided to use hydrogen peroxide to prepare gas M.
(a) Identify gas M.
(b) Mention six apparatus he can use to prepare gas M.
(c) Write the word equation for the laboratory preparation of gas M.
(d) State two uses of gas M.
9 (a) write the Dalton’s Atomic theory that lead to the following modern atomic theory.
- Atoms are made up of smaller particles called PRPTONS, ELECTRONS, and NEUTRONS.
- Atom can be CREATED or DESTROYED or SPLT up by nuclear reactions.
(b) Give three reasons to why charcoal is still being used by majority of Tanzanians for domestic purpose.
(c) Draw and write electronic configuration of the following elements
- Sodium
- Sulphur.
SECTION C (15) MARKS
10. (a) Scientific procedures are steps used by scientists when finding answers to scientific problems. Write the steps which correspond to each of the following.
- Kelvinia was not feeling well. Shewent to see a medical doctor at Malangali Health Center.
- The Doctor asked Kelvinia several questions about how she was feeling.
- The Doctor ordered Kelvinia’s body temperature, blood and urine sample for observation in the laboratory.
- The laboratory technician diagnosed Malaria parasite in Kelvinia’s blood.
- The doctor confirmed that Kelvinia had Malaria and prescribed medicine for her.
(b) Why is scientific procedure important? Give two points
(c) State three areas where scientific procedures are applied
FORM TWO CHEMISTRY EXAM SERIES 47
FORM TWO CHEMISTRY EXAM SERIES 47
TANZANIA HEADS OF ISLAMIC SCHOOLS COUNCIL
FORM TWO INTER ISLAMIC MOCK EXAMINATION
CHEMISTRY
TIME: 2:30 Hours Thursday 27th July, 2023 p.m.
Instructions
- This paper consists of sections A, B and C.
- Answer all questions in the spaces provided.
- Read carefully the instructions given in each section before answering the questions.
- All writings must be in blue or black ink except drawings which must be in pencil.
- All communication devices and calculators are not allowed in the examination room.
- Write your Candidate Number on the top right on every page.
- The following atomic masses may be used:
- H=1, C=12, O=16, N=14, S=32, Zn=65, Cl=35.5, Cu=64
| FOR EXAMINERS’ USE ONLY | ||
| QUESTION NUMBER | SCORE | EXAMINER’S INITIALS |
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 | ||
| 10 | ||
| TOTAL | ||
| CHECKER’S INITIALS | ||
SECTION A (15 Marks)
Answer all questions in this section
1. For each of the items (i) – (x), choose the correct answer from among the given alternatives and write its letter in the box provided.
(i) Form one students went to the laboratory to measure fixed volume of distilled water. Which apparatus can they use?
- Beaker
- Burette
- Pipette
- Measuring cylinder
(ii) Which one of the following state of matter its volume expands indefinitely to occupy the space?
- Liquid
- Solid
- Gas
- Solution
(iii) Different gases composing Air are present in various proportions by volume. Which among the following gases its percentage in Air is not constant and varies depending on various factors?
- Nitrogen
- Oxygen
- Noble gases
- Water vapour
(iv) What happens to an atom when it becomes an ion with a charge of +1?
- It gains an electron
- It loses an electron
- It gains a proton
- It loses a proton
(v) Musa and Juma were debating about the general group trends of properties of an element in the periodic table. What trend will you recommend to them?
- Electronegativity increases down
- Densities decrease downward
- Melting point decreases downward
- Ionization energy increases downward table
(vi) Form two students were arguing about the electronic configuration of magnesium ion. Which among the following will you suggest to them?
- 2:8
- 2:8:2
- 2:8:6
- 2:8:8:2
(vii) Mr. Mwaisa coated the roofs of his house with zinc in order to protect it from rusting. What was the process done by Mr. Mwaisa?
- Painting
- Galvanization
- Oiling
- Alloying
(viii) Which changes of state of matter take place when the temperature of a substance is lowered?
- Boiling and melting
- Condensation, freezing and melting
- Condensation and freezing
- Melting only
(ix) The teacher was demonstrating the number of electrons a Nitrogen has to lose or gain when forming Ammonium ion. What will probably be the number?
- +3
- -3
- +4
- -4
(x) Which method is used to separate a mixture of the following liquids?
| Liquid | Boiling point (oC) |
| Methanol | 64.5 |
| Ethanol | 78.5 |
| Propanol | 97.2 |
| Butanol | 117.6 |
- Crystallization
- Evaporation
- Filtration
- Fractional distillation
2. Match the uses of Hydrogen gas in LIST A with the corresponding properties in LIST B by writing the letter of the correct response beside the item number.
| LIST A | LIST B |
|
|
ANSWERS:
| LIST A | (i) | (ii) | (iii) | (iv) | (v) |
| LIST B |
SECTION B (70 Marks)
Answer all questions in this section
3. (a) Some of the elements have their symbols completely different from their common English names.
(i) Where are their symbols derived from? _______________________________
(ii) List any four (4) elements of this kind
| English name | Latin Name | |
| (i) | ||
| (ii) | ||
| (iii) | ||
| (iv) |
(b) The teacher told Anna to list two elements which she knows. Anna wrote Al and O.
Why did she use a letter instead of writing their full names? Give two (2) reasons.
(i) _______________________________________________________________
(ii) _______________________________________________________________
(c) Form one students were arguing that solutions and suspension have the same appearance. Were they correct? Give two (2) reasons.
(i) _______________________________________________________________
_______________________________________________________________
(ii) _______________________________________________________________
_______________________________________________________________
4. (a) Mr. Mjatanga was preparing food for his son using hot oils on a frying pan. Accidentally, the pan toppled over and a huge fire spread on the kitchen floor.
(i) Which fire extinguisher would be suitable for putting out the fire?
_______________________________________________________________ _______________________________________________________________
(ii) Why water would not be suitable for putting off the fire stated in 4(a) above?
_______________________________________________________________ _______________________________________________________________
(b) Give reasons to support each of the following statements:
(i) Commodities like hand bags and camera bags for sale are packed with Silical gel.
_______________________________________________________________
_______________________________________________________________
(ii) When iron sheets exposed to wet air for a longtime, they turn to reddish brown in colour
_______________________________________________________________
(iii) If the clothes worn by your friend catch fire, cover them with a fire blanket
_______________________________________________________________
_______________________________________________________________
5. (a) Elements T and Q have atomic number 12 and 17, respectively. Use the two elements to answer the following questions:
(i) Write the electronic configuration of element Q.
(ii) What is the valency of element T?
(iii) Write the chemical formula of a compound formed when T and Q combine _
(iv) Mention the type of the bond formed by the combination of element T and Q.
(v) In which group and period in the periodic table does element Q belong?
_______________________________________________________________
_______________________________________________________________
(b) The orange and yellow colour of Cr2![]() and CrO42? respectively are determined by the number of electrons lost by the chromium. Determine these numbers of electrons lost by chromium ion in each radical.
and CrO42? respectively are determined by the number of electrons lost by the chromium. Determine these numbers of electrons lost by chromium ion in each radical.
| CrO42? | Cr2O72? |
6. (a) The laboratory technician planned to label warming signs on the containers found in the laboratory. Suggest the name of warming sign he could label on the containers containing the following:
(i) Rat poison ______________________________________________________
(ii) Methylated spirit ________________________________________________
(iii) Hydrogen peroxide _______________________________________________
(iv) Concentration sulphuric acid _______________________________________
(v) Cooking gas ____________________________________________________
(b) Adil was hit on his head by Yassir in a football game between their classes. Adil lost consciousness and fainted. Being an expert, which steps you would follow to help him before taken to hospital. (Give five points)
(i) _______________________________________________________________
(ii) _______________________________________________________________
(iii) _______________________________________________________________
(iv) _______________________________________________________________
(v) _______________________________________________________________
7. A student went to the Chemistry laboratory and found reagents and apparatuses on top of the beach. List of apparatuses
(i) Thistle funnel with cork
(ii) Round bottomed flask
(iii) Delivery tube
(iv) Water trough
(v) Gas jar
(vi) Beehive shelf
(vii) Stoppers
Reagents
(i) Hydrogen peroxide
(ii) Manganese (IV) oxide
(iii) Water
He developed an idea on the laboratory preparation of gases
(a) What is the gas that is likely to be prepared using the laboratory reagents and apparatuses shown above?
_____________________________________________________________________
(b) Write a well-balanced formula equation for the reaction involved in the preparation of the gas above?
_____________________________________________________________________
(c) What are the function of manganese (IV) oxide in the preparation of the gas named above?
_____________________________________________________________________
(d) With the aid of diagram show the laboratory set up for preparation of the gas named above.
(e) State the chemical test to identify the gas prepared above.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
8. (a) At Magoza village, most of societies are using firewood and charcoal as a source of fuel for domestic use. Give three (3) reasons, why the villagers prefer to use such types of fuel?
(i) _______________________________________________________________
(ii) _______________________________________________________________
(iii) _______________________________________________________________
(b) Assess three (3) effects of using such fuels by villagers at Magoza village to the environment.
(i) _______________________________________________________________
(ii) _______________________________________________________________
(iii) _______________________________________________________________
(c) “Energy can neither be created nor destroyed, but it can be transformed from one form to another.” Which forms of energy can be transformed on the following conditions?
(i) Plants carrying photosynthesis process
_______________________________________________________________
_______________________________________________________________
(ii) Boiling water using gas cooker
_______________________________________________________________
_______________________________________________________________
(iii) Production of electricity using radioactive materials
_______________________________________________________________
_______________________________________________________________
(iv) Switching on a bulb
_______________________________________________________________
_______________________________________________________________
9. (a) Compound Q composed of 15.8% carbon and the rest is Sulphur. If the vapour density of compound Q is 38;
(i) Find the percentage of Sulphur in a compound Q.
(ii) Calculate the empirical formula of the compound Q.
(iii) Calculate the molecular formula of the compound Q.
(b) You are provided with the following chemical formulas CO2, CH4, C2H6 and NH3. Categorize the given formulas as empirical and molecular formulas.
| Empirical formula | Molecular formula |
SECTION C (15 Marks)
Answer question ten (10)
10. (a) (i) Solid, liquid and gas are the three states of matter that happen exchangeably. Identify one important factor that govern changes among them.
_______________________________________________________________
_______________________________________________________________
(ii) State at least one method of separating mixture that can be useful to obtain one of the following substances:
1. Kerosene mixed with water _____________________________________
____________________________________________________________
2. Iodine from sand ______________________________________________ ____________________________________________________________
3. Ethanol from water ____________________________________________
____________________________________________________________
4. Cooking sunflower oil from sunflower seeds _______________________
____________________________________________________________
(b) Life without changes of state of matter could be almost impossible. Defend this fact by giving five (5) points.
(i) _______________________________________________________________
_______________________________________________________________
(ii) _______________________________________________________________
_______________________________________________________________
(iii) _______________________________________________________________
_______________________________________________________________
(iv) _______________________________________________________________
_______________________________________________________________
(v) _______________________________________________________________
_______________________________________________________________
(c) Identify the following properties of flames into luminous flame and non-luminous flame in the following:
| S/N | PROPERTIES OF FLAME | TYPE OF FLAME |
| 1 | Is not very hot (produce less heat) | |
| 2 | Is not sooty | |
| 3 | Is bright yellow in colour | |
| 4. | Is very hot (produce more heat) | |
| 5 | Produce more light |
FORM TWO CHEMISTRY EXAM SERIES 42
FORM TWO CHEMISTRY EXAM SERIES 42
PRESIDENT'S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
KILIMANJARO REGION MOCK FORM TWO EXAMINATION
CODE:032 CHEMISTRY
TIME: 2:30 HOURS MAY 2023
INSTRUCTIONS
- This paper consists of section A and B with total of ten (10) questions.
- Answer all questions in the space provided.
- Section A and C carry Fifteen (15) marks each and section B carries Seventy (70) marks.
- All Writing Must be in black or blue ink except diagrams which must be in pencil.
- Any unauthorized Materials are not allowed in the examination room.
- Write your name at the top right corner of every page.
| FOR EXAMINER'S USE ONLY | ||
| QUESTION NUMBER | SCORE | INITIALS OF EXAMINER |
| 1. | ||
| 2. | ||
| 3. | ||
| 4. | ||
| 5. | ||
| 6. | ||
| 7. | ||
| 8. | ||
| 9. | ||
| 10. | ||
| TOTAL | ||
SECTION A (15 MA R KS)
Answer all questions in this section
l . For each of the items (i-x) choose the correct answer among the given alternative and write its letter in the box provided.
(i) Which term describes a rapid chemical reaction that release energy in the form of heat and light
- Ignition
- combustion
- Heating
- Flame
(ii) The unburnt region of the non-lunlinous flame is . . . . . . . in colour
- Colourless
- Black
- Pale blue
- Green
(iii) Why hydrogen is not a constituted of air?
- Because of being highly flammable
- Because of being a reducing agent
- Because of being very light
- Because of being very reactive
(iv) Which of the following warning signs is likely to appear on the bottle containing concentrated Nitric acid in the laboratory?
- Explosive
- Flammable
- Harmful
- Corrosive
(v) How can one prevent rusting in fragile instrument like camera?
- By using silica gel
- By using ethanol
- By galvanization
- By using oil
(vi) The teacher was demonstrating an experiment by dissolving sodium chloride in water until the solute was not dissolving any more. What type of' solution was formed at the end of experiment
- Saturated
- Unsaturated
- Suspension
- Super saturated
(vii) What does the random movement of grains suspended in air demonstrates?
- Matter is solid in nature
- Matter is solid, liquid or gas in nature
- Matter is Particulate in nature
- Matter is gaseous in nature
(viii) A chemist observed that when iron nail is left overnight the nail rust. He later said that this was due to presence of moisture in the atmosphere during the night. This can be termed as:
- Hypothesis
- Observation
- An interpretation
- Conclusion
(ix) Which of the following are the products of the reaction of sodium with water?
- Sodium oxide and hydrogen gas
- Sodium oxide and water vapour
- Sodium hydroxide and hydrogen gas
- Sodium hydroxide and water vapour
(x) Which of the following electronic configurations are of metal?
- 2:8:8:1 and 2:8:8:7
- 2:8:3 and 2:8
- 2:8:8:2 and 2:8:3
- 2:8:6 and 2:8:4
| i. | ii. | iii. | iv. | v. | vi | vii | viii | ix | x. |
| | | | | | | | | | |
2. Match each item in list A with correct response in list B by writing the letter of' the correct response below the corresponding item number in the letter provided'
| LIST A | LIST B |
| (i) A condition in which the body system is unable to take blood to the vital organs. (ii) A skin injury caused by hard hit. (iii) Sudden loss of consciousness caused by lack of sufficient blood and oxygen to the brain. (iv) Blockage of the upper part of the airways by food. drink or other objects. (v) A condition in which the lungs are not getting enough oxygen causing difficulty in breathing |
|
ANSWERS
| List A | i. | ii. | iii. | iv. | v. |
| List B | | | | | |
SECTION B (70 Marks)
Answer all questions in this section
3. (a) Chemistry plays great role in our world. Its knowledge and skills applied when solving daily life problems related to food, water, soil. health. energy, shelter, clothing, and clean air. Justify this fact by giving five importance of studying chemistry.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Explain live areas where chemistry is applied.
| Area | Application |
| i. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | i. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| ii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | ii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| iiii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | iii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| iv. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | iv. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| v. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | v. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4. (a) One of the significant of chemical symbols is to distinguish one element from the other and quickly understand the elements being referred to assign chemical symbol for each of the following elements.
| Element | Chemical symbol |
| i)Copper | |
| ii) Carbon | |
| iii) Calcium | |
| iv) Chlorine | |
| v) Cobalt | |
(b) Differentiate luminous flame from non-luminous flame by giving five points.
| Luminous flame | Non-luminous flame |
| i. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | i. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| ii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | ii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| iii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | iii. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| iv. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | iv. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
| v. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | v. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5. Study the periodic table for the first 20 elements below
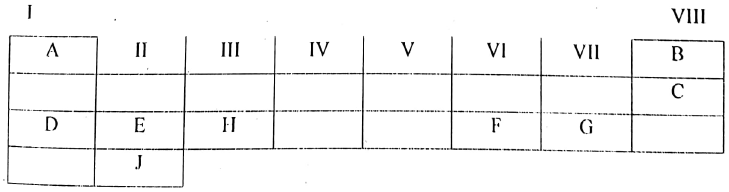 |
Use the letters shown in the periodic table above to indicate:
- The lightest atom
- An alkaline earth metals
- A halogen
- Element with O valence, used to fill weather balloons
- Write the electronic configuration of J
- Give the names of A and F
- Write the chemical formula when H combine with F
6. (a) Fuels may be classified on the basis of their physical state with one example, give the three main states Fuels.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) There is a close relationship between some physical properties of water and usefulness. State two main physical properties of water and their usefulness.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7. Halima needed to measure the volume of water in the chemistry laboratory. Which apparatuses should be used.(three apparatuses)
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Name three apparatuses that are made by ceramic materials
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) During practical activities in the laboratory several accidents could occur. Mention four accidents that needs first aid procedures
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
8. (a) By giving a reason, suggest the suitable method of separating each of the following
| Method | Reason | |
| i. Ethanol and water | ||
| ii. Cooking and water | ||
| iii. Ammonium chloride and sand. |
(b) Briefly explain each of the following phenomena
- A black copper (iii) oxide changed to brown colour when combine with hydrogen gas
- A bottle fully of water burst when exposed in a deep freezer overnight
9. (a) Assign each of the following changes to either a physical change or a chemical change by putting a tick on the respective column in the following table.
| Change | Physical chance | Chemical change |
| i. Iron sheets rust | | |
| ii. Water evaporates from the surface of ocean | | |
| iii. D in of wet clothes | | |
| iv. Milk turn sour | | |
| v. Butter melts on warm toast | | |
b) If a sample X consists of 99.76% of 16X, 0.04% of 17X and 0.3% of 18X
- Calculate the relative atomic mass of x.
- Identify the element X.
SECTION C (15 Marks)
Answer question 10
10. (a) A chemistry teacher wants to demonstrate the laboratory preparation of oxygen by decomposition method. He appointed a form two students to collect apparatus for this experiment.
- List all apparatus for this experiment above.
- State two reagents for the laboratory preparation of' oxygen by decomposition.
(b) Write a word equation for the reaction between two reagents above.
(c) State five physical properties of the gas produced above.
FORM TWO CHEMISTRY EXAM SERIES 33
FORM TWO CHEMISTRY EXAM SERIES 33
THE UNITED REPUBLIC OF TANZANIA PRESIDENT’S OFFICE - REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
LUDEWA DISTRICT COUNCIL
FORM TWO MOCK EXAMINATION 2022
032 CHEMISTRY
TIME: 2:30 Hours 8th JUNE 2022
INSTRUCTIONS
i. This paper consists of section A, B and C
ii. Answer All questions in all sections
iii. Calculators and cellular phone are Not allowed in examination room
iv. Write your number on every page on every page on space provided
v. Constants used;-
H=1, C=12, O=16, Ca=40, Cl=35.5, K=39 ,Na=23 , N=14
SECTION A (15 Marks)
Answer all questions from this section
1. From item (i-x), choose the correct item from the given alternatives and write its answer beside the item number provided.
i. A form two student was preparing different types of mixtures in the chemistry laboratory. The first mixture, he dissolved 30grams of sodium chloride crystals (NaCl) in 500cm3 of water then he stirred the mixture for about five minutes. What is the name of this mixture prepared by a student?
- Heterogeneous mixture
- Suspension mixture
- Colloidal mixture
- Homogenous mixture
ii. One student from form one observed that most of the kitchen tools such as plates, cups, bowl and jugs are made of steel which is smooth and shiny. As a chemistry student why these kitchen tools are made up by such steel?
- To make them look expensive
- To make them shiny
- To prevent them from rusting
- To make easy to see
iii. Kimbe was doing experiment concerning flame test of different chemical substances in the chemistry laboratory, unfortunately his laboratory coat caught fire .Which of the following fire extinguishing items will you use to help him to extinguish fire from his laboratory coat?
- Fire blanket
- Carbon dioxide
- Water
- Form
iv. An element H with valency of 2 react with another element L with valency of 3. What is the probable chemical formula between atoms of element H and element L?
- H2L3
- L2H3
- H3L2
- HL2
v. Jackline when was mopping chemistry laboratory she saw a sign of cross on a plastic bottle containing a certain chemical. Which of the following is the correct answer about the interpretation made by Jackline concerning the property of chemical contained in the bottle?
- Radioactive
- Harmful
- Toxic
- Oxidant
vi. Form four students in a certain school were conducting experiment about volumetric analysis. In their experiment they were required to transfer three drops of methyl orange from the reagent bottles into the reacting vessels. Do you think which of the following apparatus they were using to transfer three drops of methyl orange from reagent bottle to reacting vessels?
- Beaker
- Burette
- Spatula
- Dropper
vii. Students from Lugarawa health training center were doing a scientific research about malaria in Njombe region. Which among the following scientific procedures they were using to accept or to reject their hypothesis made?
- Conclusion
- Data interpretation
- Data analysis
- Experimentation
viii. During the practical session one student mixed accidentally the iron fillings with sulphur powder. Assume you are the expert of chemistry which method will you use to obtain these two components of the mixture separately?
- Piking
- Filtration
- Magnetization
- Evaporation
ix. Boron is known to have two stable isotopes of atomic masses 10.016g and 11.013g. The isotopes normally exists in nature in the proportions of 20% and 80%respectively, from the data what is the average mass of Boron?
- 10.81g
- 10.62g
- 10.51g
- 10.58g
x. Laboratory technician wanted to know the liquid present in beaker labeled L left after experiment. He took two drops of liquid L and he decided to add it into petri dish containing white anhydrous copper (II) Sulphate crystals. Immediately after the addition of three drops of liquid L into petri dish containing the anhydrous copper (II) Sulphate crystals the mixture turned to blue colour. What is the name of liquid present in beaker labeled L?
- Water
- Acid
- Salts
- Base
ANSWERS
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
2. Match the items in list A with the responses in list B by writing the letter of the correct response beside the item number in the answer booklet provided.
| List A | List B |
| i. Means that a substance is dangerous and can cause death within a short time ii. Means that a substance is dangerous and can affect our health for long time iii. Means that the substance emits harmful radiations that penetrate human body and cause damage. iv. Means that the substance can speed up the rate of burning. v. Means that the substance can catch fire easily. |
|
ANSWERS
| LIST A | i | ii | iii | iv | v |
| LIST B |
3. a) Most of farmers in the rural areas were interested to know how the knowledge of chemistry is necessary in agricultural activities. Assume you are a chemist; educate these farmers on the application of chemistry in agriculture by giving five points.
b) The headmaster of a certain school was bought a small box which had a sign of Red Cross. Most of form one students they did not have a knowledge about that box.by using knowledge that you have learnt from chemistry answer the following questions.
i. What is the name given to that box
ii. Briefly explain the functions of four possible items that can be found in a box
4. a) A laboratory technician advised students to use non-luminous flame when they are heating chemicals in the laboratory. As a form two student explain briefly four reasons why he encouraged students to use this kind flame?
b) Some people in the society believe that solving problems by using scientific procedure is the wastage of time and money. By using knowledge of chemistry that you have refute the statement by giving four points.
5. a) Assume you are given two gas jars, one gas jar containing gas A and another gas jar containing gas B. Gas A is used to fill balloons while gas be is used to by ocean deep divers.
i. What are the chemical test of gas A and gas B?
Gas A . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Gas B . . . . . . . . . . . . . . . . . . . . . . . . . . . .
ii. Explain five uses of gas A in your daily life.
b) Mwahisa placed a yellow solid on a deflagrating spoon, ignited and then lowed the spoon into a gas jar of oxygen .The solid burnt vigorously with a yellow flame and finally the product was a pale yellow solid.
i. What is the name of the yellow solid that is burnt in oxygen
ii. Write the chemical formula of the pale yellow product formed
iii. Write the word equation for the reaction
6. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
7. a) A form one student from a certain school given a task by her teacher to state the type of fire extinguisher(s) that she will use to extinguish fire caused by the burning materials listed below. Unfortunately a student failed to attempt this question correctly. As a form two student help her to answer this question.
i. Burning curtain
ii. A fire started by a short circuit in an electric iron
iii. A fire started by hot oil in a frying pan
iv. Fire started on a bucket containing sodium metal
b) Cars, motorcycles and corrugated iron sheets in coastal towns like Dar es Salaam, Tanga and Zanzibar are found to rust more quickly than in other cities in Tanzania like Songea, Njombe, Mwanza and Arusha. Why is this so?
8. a) A compound consists of 27% sodium, 16.5% nitrogen and 56.5% oxygen by mass. Calculate the simplest formula of the compound.
b) During the practical session, Asha found some bottles in the laboratory containing different chemical compounds with different chemical formulas, unfortunately she failed to write the IUPAC names of these chemical compounds. As form two student help her by writing the correct IUPAC names of these chemical compounds found by Asha on bottles in the laboratory.
i. KCl . . . . . . . . . . . . . . . . . .
ii. Na2SO4 . . . . . . . . . . . . .
iii. CaCl2 . . . . . . . . . . . . . . .
iv. AlCl3 . . . . . . . . . . . . . . .
v. SF6 . . . . . . . . . . . . . . . . .
9. a) Oxygen exists naturally in three different isotopes which are, 168O(99.76%), 178O(0.04%) and 188O(0.20%). Calculate the relative atomic mass of oxygen
b) Use the knowledge that you have complete the following table below by indicating the number of protons, electrons and neutrons of different atoms shown in the table below. The atomic number and mass number are given.
| No | Atom | Atomic number | Mass number | Protons | Electrons | Neutrons |
| i. | Beryllium | 4 | 9 |
|
|
|
| ii. | Fluorine | 9 | 19 |
|
|
|
| iii. | Sodium | 11 | 23 |
|
|
|
| iv | Oxygen | 8 | 16 |
|
|
|
SECTION C: 15 MARKS
10. Nowadays the world is suffering from climatic changes which is caused by over dependent on forests as a source of fuels, and over production of waste materials that pollute our environment. To overcome this problem is through the use of renewable source of energy such as biogas produced from animal dungs and sewage wastes. As the expert of chemistry prepare a report to convince the community to use this gas produced by animal dungs as the alternative source of energy while they are conserving the environment.
FORM TWO CHEMISTRY EXAM SERIES 25
FORM TWO CHEMISTRY EXAM SERIES 25
Students Assesment Number_________________
THE PRESIDENT’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
MOSHI MUNICIPAL COUNCIL
SECONDARY EDUCATION EXAMINATIONS SYNDICATE
032 CHEMISTRY
FORM TWO MOCK ASSESMENT
Tuesday 28th June 2022 Am Time 2:30Hours
Instructions
1. This paper Consist of sections A, B and C with total of 10 questions
2. Answer all questions in both sections
3. All writings should be in blue/black ink
4. All diagrams should be drawn in pencil
5. Write your assessment number at the top right corner
SECTION A (15Marks)
1. For each of the items (i)-(x), choose the correct answer from among the given alternative and write its letter in the box provide.
i. The welders prefers to use non luminous flame for their work simply because
- It is available
- it is easy to transport
- produce very hot flame
- can be made by kerosene
ii. Spatula in the laboratory is used for scoping what types of substances?
- Liquid and gases
- solids and liquids
- Powdery and gases
- Solids and powdery
iii. There are two particles inside the nucleus which one contributes the net change of that nucleus?
- Dalton
- Protons
- Electrons
- Neutrons
iv. Why oxygen as one of the components of air Is unique?
- It has ability to burn
- it support combustion
- it is diatonic gas
- combine with carbon dioxide
v. Why it is necessary to boil drinking water?
- To make it tasteless
- To remove impurities
- to kill micro-organism
- To make it taste less
vi. How do chemists refer to a mixture of milk and water?
- Miscible solution
- Suspension
- Immiscible solution
- Emulsion
vii. How can one prevent rusting in fragile instruments like camera?
- By galvanization
- By using silica gel
- By using oil
- By using ethanol
viii. The oxidation state of metallic element is always
- Negative
- Positive
- Zero
- Neutral
ix. Which of the following is not man-made product of applying chemistry?
- Sugar
- Fertilizer
- Milk
- Vaccines
x. The atomic number of element R is 9 What is its electronic configuration?
- 2:4:3
- 2:5:2
- 2: 7
- 2:6:1
ANSWERS
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
2. (a) Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided
| List A | List B |
|
|
ANSWERS
| i | ii | iii | iv | v |
SECTION B: (70MARKS)
3. (a) Mrs. Msema kweli was addressing the villagers about use of water for economic values. What 4 values will she tell the villagers
(b) Is an air a mixture or a compound? Give 4 reasons
4. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv.Sterile gauze
5. (a)State three sub- atomic particles and their respective charge.
(b)State the four modification of Dalton`s atomic theory
(c)Draw and write the electronic configuration for the atoms having the following atomic numbers.
(i) 4Be
(ii)14Si
6. (a)Distinguish the following substances.
i. Homogenous mixture from heterogeneous mixture.
ii.Miscible from immiscible liquids.
iii. Saturated from unsaturated solution.
(b)Give two chemical tests for pure water
7. (a)Asha want to select a good fuel for domestic uses in her home what things should she considered? (Mention six (6)
(b)Classify the following fuel either primary or secondary fuel.
(i) Coke
(ii)Crude oil
(iii)Kerosene
(iv)Natural gas
8. (a)What properties of hydrogen gas that made it to be used in the following?
i. As a fuel
ii. To fill weather balloons
iii. Manufacture of hydrochloric acid
iv. Manufacture of margarine
(b) Environmental pollution in most of rural areas in Tanzania is caused by using charcoal and firewood as a fuel. What will be the two alternative source of energy, they supposed to use for environmental conservation? (give reason for each)
(c) Why fossil fuels are referred to as non-renewable energy resources? (Give two reasons)
9. (a)You are provided with the following atoms, for each fill the informations below:-
(i) 35.5Cl
protons =
Neutrons=
Electrons=
(ii) 1H
protons =
Neutrons=
Electrons=
(b) Define the following
i.Atom
ii. Energy
iii.Atomic number
v. Isotopes
SECTION C (15 MARKS)
10a)i. What do you understand by the word “Relative atomic mass”?
ii). Carbon has main isotopes,126C and 146C with relative abundances 98.89% and1.11% respectively. Calculate the relative atomic mass of Carbon.
b)i. Draw a diagram of laboratory preparation of oxygen gas from hydrogen peroxide and manganese IV oxide
(ii) Write the word equation for the reaction above (a)i
c) Answer the following questions with reference to the first 20 elements of the periodic table
i. Give the chemical symbol of element having:
• The smallest atomic size______________________________________________
• The largest atomic size _______________________________________________
ii.Identify the element which are:
• Metals having three shells of electrons each
• Metals having 1 electron in the valence shell
• Noble gases ___________________________________________________________
FORM TWO CHEMISTRY EXAM SERIES 16
FORM TWO CHEMISTRY EXAM SERIES 16
PRESIDENT’S OFFICE REGIONAL ADMINISTRATIVE AND LOCAL GOVERNMENT
TANZANIA HEADS OF ISLAMIC SCHOOLS COUNCIL
FORM TWO INTER ISLAMIC MOCK EXAMINATION
032 CHEMISTRY
TIME: 2:30 HOURS Monday, 27th September 2021 p.m.
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.
2. Answer all questions in the spaces provided.
3. All writings must be in blue or black ink except drawings which must be in pencil.
4. All communication devices and calculators are not allowed in the examination room.
5. Write Your Examination Number on the top right of every page.
6. The following atomic masses may be used.
H = 1, C = 12, O = 16, N = 14, S = 32, Zn = 65, Cl = 35.5, Na = 23.
| FOR EXAMINER’S USE ONLY | ||
| QUESTION NUMBER | SCORE | EXAMINER’S INITIALS |
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 | ||
| 10 | ||
| TOTAL | ||
| CHECKER’S INITIALS | ||
SECTION A: (20 Marks)
1. Write down the letter corresponding to the most correct answer in the box provided for each item.
(i) How many protons, neutrons and electrons are there in an atom represented by ![]() ?
?
| Protons | Neutrons | Electrons | |
| A. | 35 | 17 | 18 |
| B. | 17 | 18 | 17 |
| C. | 18 | 17 | 35 |
| D. | 17 | 35 | 18 |
(ii) Which of the following methods is used to separate salt from sea water?
- Filtration
- Decantation
- Decantation
- Fractional distillation.
(iii)Which of the following warning signs is likely to appear on a gallon containing methylated spirit in the laboratory?
- Irritant
- Flammable
- Oxidant
- Explosion.
(iv) Hydrogen peroxide ![]() (iv) X + water. “X” represents _________
(iv) X + water. “X” represents _________
- Oxygen
- Hydrogen
- Nitrogen
- Carbondioxide.
(v) If a Bunsen burner produces much soot, which is the correct conclusion?
- The air hole is closed
- The burner gas jet is big
- The air hole is fully opened
- The gas supply is poor.
(vi) Which of the following is the list of group (II) element?
- Sodium and magnesium
- Calcium and carbon
- Magnesium and calcium
- Boron and Beryllium.
(vii) Element M of group I combines with element X of group VI, the formula of the compound formed when M combines with X is:
- MX2
- MX6
- X2m
- M2X.
(viii) The pair of elements which is most likely to form a covalent bond when reacted together is:
- Carbon and chlorine
- Aluminium and oxygen
- Magnesium and oxygen
- Sodium and chlorine.
(ix) One of the following can distinguish hydrogen gas from other gases:
- It burns and supports combustion
- It is colourless and odorless
- It gives a smell of a rotten egg
- It burns with pop sound.
(xi) The following sentences suggest that air is a mixture, except:
- Its components can be separated physically
- Its composition varies from one place to another
- Its properties depends on individual gases
- Its formation requires absorption or reabsorption of heat.
2. (a) Choose a word(s) from list B which matches the statement or phrases in list A and write its letter in the space provided.
| LIST A | LIST B |
| (i) Factors that can be manipulated to get desired results (ii) A factor that is kept constant (iii) Scientist’s best possible answer (iv) Statement of how the results related to hypothesis (v) First step in scientific method of studying a problem |
|
Answer:
| List A | (i) | (ii) | (iii) | (iv) | (v) |
| List B |
2. (b) Fill in the blanks below with the correct words.
(i) A candle flame is not good for glass ware because _____________________
(ii) A catalyst used for the production of oxygen gas from hydrogen peroxide is _______________________
(iii) A sudden unexpected occurrence that destroy properties and cause injury is called __________________________
(iv) It is used as fuel in rockets due to its lightness _________________________
(v) Blockage of the upper part of airway by food or other objects is called ________________________________
SECTION B: (80 Marks)
Answer all questions in this section
3. (a) What is fuel?
(b) (i) State four types of fuels used by many Tanzanians:
(ii) Why do you think many Tanzanians prefer to use the mentioned sources in 3b(i) above? Give two best reasons.
4. You are provided with the following materials from chemistry laboratory:
Apparati: Thistle funnel, delivery tube, beehive self, trough, gas jar, flat bottomed flask with its coke.
Chemicals:Water, zinc granules, dilute hydrochloric acid.
(a) What chemical substance can be prepared using the materials mentioned above?
(b) Draw the diagram showing how you could arrange the Apparati above in order to prepare a chemical substance you mentioned in 4(a) above. 05 marks
(c) How can the products mentioned in 4(a) above be collected?
(d) Give at least three (3) uses of the product you mentioned in 4(a) above based on its properties.
5. (a) What should you do immediately if:
(i) The piece of broken beaker cuts your finger
(ii) Chemicals splash on your face
(iii) Your shirt has caught fire
(iv) Your fellow student swallows unknown chemical substance thinking that it was water
(b) Why do you think first aid is an important thing? Give two points:
6. (a) (i) A table salt is a common name for the compound with the formula NaCl.
Write the systematic name for the table salt
(ii) Briefly describe why molecular formula better preferred than empirical formula is
(b) A compound consists of 27% sodium, 16.5% nitrogen and 56.5% oxygen by mass. If its molecular mass is 85g, find:
(i) Its empirical formula 05 marks
(ii) Molecular formula 02 marks
7. (a) Briefly explain the following terms:
(i) Periodicity
(ii) Electronegativity
(iii) Ionization energy
(iv) Atomic radii/size.
(b) Why are certain elements in the period table referred to as metalloids? Give two examples of such elements:
Reason: ____________________________________________________________
02 marks
Example:
(i) _____________________________________________________________
(ii) _____________________________________________________________
@ 00 ½ mark
(c) Element X has atomic number of 19 and neutron number 20. Write down its:
(i) Electronic configuration
______________________________________________________________
01 mark
(ii) Mass number 02 marks
_______________________________________________________________
_______________________________________________________________
_______________________________________________________________
8. (a) (i) Differentiate combustion from rusting.
_______________________________________________________________
_______________________________________________________________
(ii) Explain why cars and corrugated iron sheets in coastal areas like Dar es
Salaam, Tanga and Zanzibar get rust more quickly than in other cities of
Tanzania like Mbeya, Mwanza and Arusha? 02 marks
_______________________________________________________________
_______________________________________________________________
(b) Explain how you can put off each of the following types of fire
(i) A fire started by petrol in a container 02 marks
_______________________________________________________________
_______________________________________________________________
(ii) A fire started by a short circuit in an electric wire 02 marks
_______________________________________________________________
(iii) A fire caused by a burning magnesium metal 02 marks
_______________________________________________________________
9. (a) With an example give the meaning of the following terms as applied in Chemistry:
(i) A radical 02 marks
_______________________________________________________________
_______________________________________________________________
(ii) Oxidation state 02 marks
_______________________________________________________________ _______________________________________________________________
(b) Find the oxidation numbers of the following underlined elements:
(i) Na 01mark
(ii) HNO3 01mark
(iii) H2SO4 01mark
(iv) SO32- 01mark
(c) Draw diagrams showing the electron transfer in the formation of MgCl2.
10. (a) Differentiate the following terms:
(i) An element and an atom
(ii) A compound and a mixture
(b) Melting of ice and burning of papers are two processes that happen almost daily in our life.
(i) Identify the two actions as being physical or chemical change.
- Ice melting __________________________________________________
- Paper burning ________________________________________________
(ii) Mention two significant things that happen when the paper is burnt:
(c) What are the names of elements represented by the following symbols?
(i) K _________________________________________________________
(ii) S _________________________________________________________
(d) Why some elements have their symbols written in one letter while others have two letters? (e.g. N and Na)
FORM TWO CHEMISTRY EXAM SERIES 12
FORM TWO CHEMISTRY EXAM SERIES 12
PRESIDENT’S OFFICE REGIONAL ADMINISTRATIVE AND LOCAL GOVERNMENT
KILOSA DISTRICT COUNCIL
FORM TWO TERMINAL EXAMINATION
CODE: 032 CHEMISTRY
TIME: 2.30Hours. Tuesday August 25, 2020.
Instructions
• This paper consists section A and B with a total of ten (10) questions
• Answer all questions in the spaces provided.
• All writing must be in black or blue ink except diagrams which must be in pencil.
• The following atomic masses may be used: N= 14, C= 12, H= 1, S= 32, O= 16, Cu= 63,Cl=35.5
SECTION A (20 MARKS)
Answer all questions in this section
1.This section consists of multiple choice items (i)-(x).Write down the letter of the most correct response for each question:
(i) Chemistry is one of the sciences which deals with:
- The study of body cells
- Composition,properties and behavior of matter
- Chemical changes
- Physical changes
(ii) The process of chlorination in water treatment aims at:
- Syrup making
- Removing bad smell
- Killing micro-organisms
- Formation of suspension
(iii) The atomic number of an element is the:
- Number of protons
- Mass number
- Number of neutrons
- Number of protons and neutrons
(iv) The substance that can burn your skin is best described as:
- Flammable
- Corrosive
- Toxic
- Explosive
(v) Coloured substances can be separated through the process called:
- Distillation
- Sublimation
- Filtration
- Chromatography
(vi) When you melt a piece of iron ,it undergoes:
- Combination
- Physical change
- Chemical change
- Sublimation
(vii)When element R of Group I combines with element F of Group VI, the formula of compound formed is:
- R2F
- F2R
- RF2
- RF
(viii) The apparatus which used to measure a fixed volume of 20cm3 or 25cm3 in the laboratory.
- Measuring cylinder
- Beaker
- Burette
- Pipette
(ix) The electronic configuration of sulphur is :
- 2:8:6
- 2:8
- 2:7
- 2:8:5
(x) When oxygen combines with metals they:
- Form properties
- Water
- form metallic oxides
- Rust
| (i) | (ii) | (iii) | (iv) | (v) | (vi) | (vii) | (viii) | (ix) | (x) | |
| | | | | | | | | | |
2.(a) Match each item in List A with a correct response in List B by writing its letter below the number of the corresponding item in the table provided.
| LIST A | LIST B | |||
| (i) Is a row in the periodic table (ii) Is a vertical column in the periodic table. (iii) Is the recurrence of similar properties when elements are arranged according to their atomic number. (iv) The properties of elements are a periodic function of their atomic number. (v) The collective name of the Group VII is called. |
| |||
(b). Fill in the blanks with the correct answer.
(i) ………………Involves covering of iron with zinc layer by dipping in molten zinc.
(ii) A bond formed by electron sharing………………
(iii) …………… is a substance which cannot be split into two or more simpler substance by any chemical means.
(iv) Matter is made up of very tiny particles called ................................
(v) .......................supports the bunsen burner making it stable.
SECTION B (80 MARKS)
Answer All question in this section
3. (a) Define the following terms
(i) Compound
(ii) Mixture
(b) Write the names of the processes of changing of matter from one state to another:
(i) Liquid to Gas is...............................
(ii) Solid to Liquid is .........................
(iii) Gas to Liquid is ............................
(c). Give three difference between muddy water and carbondioxide
| | Muddy water | Carbon dioxed |
| (i) | | |
| (ii) | | |
| (iii) | | |
4. Study the diagram carefully and then answer the questions that follows.
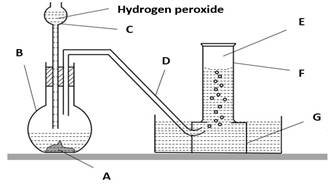
(a) (i) Name the apparatus labelled B, C, D and G.
B……………….................................................................
C………………………………………………………….
D………………………………………………..................
G……………………………………………………..……
(ii) Name the gas E ………………………………………………….
(iii) What is the test of gas E ………………………………………
(iv) Give the function of each of the following
A…………………………………………………………………
F…………………………………………………………………
(b) Give the reason to why hydrogen is used in
(i) Manufacture of margarine……………………………………….
(ii) Filling Weather balloons………………………………………
5. (a) Define the term fuel..........................................................................
……………………………………………………..……………………
(b) 2210KJ was liberated when 0.05Kg of a biodiesel was heated. Calculate the energy value of the fuel (biodiesel).
6. (a) Write the chemical formula for each of the following compound
(i) Dinitrogen trioxide
(ii) Aluminium oxide
(iii) Iron(iii) cloride
(iv) Nitric(v) acid
(c) Give the IUPAC system name of the following chemical compounds:
(i)HClO3………………………………………………
(ii) CuO………………………………………………..
(iii) P2O5………………………………………………
(c)Draw and give function of the following apparatuses:
| Apparatus | Drawing | Function |
| Measuring cylinder | | |
| Beaker | | |
7. (a) Give the meaning of the following;
(i) Radical
(ii) Valence
(iii) Oxidation number
(b) Find the oxidation state of the underlined element in the following compounds:
| (i) KClO3 | (ii) Na3PO4 | (iii) SO42- |
| | | |
8. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
9. (a) Mr. Shing’weng’we took two beakers containing water and labeled the as beaker A and beaker B. He put one spoon of chalk powder in beaker A then he stirred to mix it and in beaker B he put one spoon of sugar and stirred to mix it. Give three differences between the mixture in beaker A and the mixture in beaker B.
| S/N | MIXTURE IN BEAKER A | MIXTURE IN BEAKER B |
| (i) | | |
| (ii) | | |
| (iii) | | |
(b) Name three different kinds of natural water:
(c) State three(3) uses of water in economic activities:
10.(a) Define the following terms:
(i) Empirical formula
(ii) Molecular formula
(b) A certain compound has a molecular mass of 60g and has the following percentage composition by weight: carbon 40%, hydrogen 6.67% and oxygen 53.3%. Calculate
(i)The simplest formula of the compound:
(ii) The molecular formula
FORM TWO CHEMISTRY EXAM SERIES 2
FORM TWO CHEMISTRY EXAM SERIES 2
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256








