- Water molecules have stopped changing into ions.
- Water molecules have all changed into ions
- Concentrations of water molecules and ions are equal
- Concentrations of water molecules and ions are constant
- Water molecules are moving slow.
2
In the graph below, curve 1 was obtained from the decomposition of 100 cm3 of 1.0M hydrogen peroxide solution catalysed by manganese (IV) oxide, 2H2O 2?2H2O+O2 .
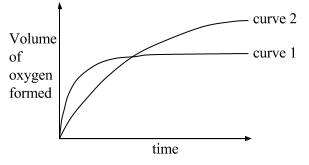
Which alteration/change to the original experimental conditions would produce curve 2?
- Lowering the temperature
- Using less manganese IV oxide
- Increasing the temperature
- Adding some 0.1 M H2O
- Using a different catalyst.
3
† Consider the system at equilibrium: H2O(l) ![]() H2O(g) for which
H2O(g) for which 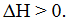 Which change(s) will increase the yield of H2O(g).
Which change(s) will increase the yield of H2O(g).
- Increase in temperature
- Increase in the volume of the container
- Increase in temperature and volume of the container
- Increasing surface area of oxygen
- Increasing surface area of reactants.
4
A catalyst can be described as a substance†
- that alters the rate of reaction
- †that slows down the rate of reaction
- †used in every reaction so as to speed up rate of reaction
- †that starts and speeds up the rate of reaction
- †that terminates chemical reaction.
5
2. Match the effects on the rate of chemical reactions in List A with the corresponding physical conditions in List B by writing the letter of the correct response beside the item number in the answer booklet provided.
| List A | List B |
| (i) Increases colliding particles per time (ii) Favours endothermic reaction (iii) Increases the speed to reach equilibrium (iv) Favours the side with fewer molecules (v) Favours more products on opposite side |
|
6
4. Zinc granules were placed in a beaker containing excess dilute sulphuric acid standing on a direct reading balance. The mass of the beaker and its contents were recorded after every two seconds as shown in Table l .
Table 1
| Time (s) | 0 | 2 | 4 | 6 | 8 | 10 |
| Mass (g) | 110.20 | 110.10 | 110.00 | 108.50 | 107.20 | 107.20 |
(a) Why there was a loss in mass?
(b) Why did the mass remain constant after the eight seconds?
(c) Briefly explain what would happen to the rate of reaction if zinc powder was used instead of granules.
View Ans7
The following equation shows the reaction between hydrogen and iodine gas to form hydrogen iodide gas,H2(g) + I2(g) ? 2HI (g) ?H= -800Kj/mol. Giving a reason, explain what would happen to the position of equilibrium if
(i) temperature is lowered.
(ii)hydrogen iodide gas is pumped into the system.
View Ans8
John measured the volume of gas produced when 5 g of two chemicals X and Y were added separately to hydrogen peroxide under identical conditions. His results for this experiment are represented on the graph below.
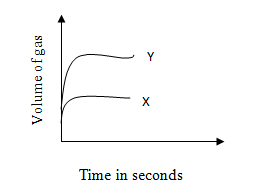
John claimed that Y is a better catalyst than X. His partner Steven did not agree.
-
Why does Steven think that Johnís conclusion is wrong?
-
After the experiment, Steven recovered 5 g of X and 1 g 1 of Y from the two experiments. He claimed that John was wrong. Does Stevenís claim true? Give a reason.
9
† Carbon monoxide and hydrogen are used in the manufacture of methanol and the equilibrium is established according to the following equation.
†CO(g) + 2H2(g) ? CH3OH(g) ?H=-80 kJ mol-1.
(i)†Give two features of the reaction at equilibrium.
(ii)†Explain why an increase in temperature causes a decrease in equilibrium yield of methanol.
View Ans10
Two experiments were carried out using the same mass of magnesium ribbon and the same volume of acids of the same concentration. The acids were 1M hydrochloric acid and 1M ethanoic acid. The results were as shown in the following figure:
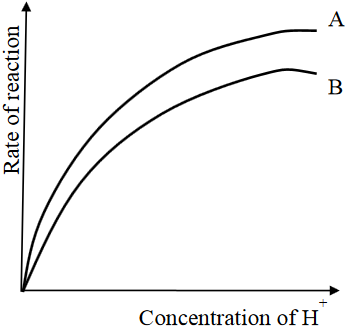
†(a)†If the experiments were conducted within the same time, is there a difference in volumes of hydrogen gas collected at the same room temperature and pressure? Give reasons for your answer.
†(b)†When same mass, volume and concentration of powdered magnesium and ethanoic acid are allowed to react, new graph is formed. Giving reason (s), suggest the position of that graph whether will be above, between or below graphs A and B.†
View Ans11
9. Two experiments A and B were conducted to prepare hydrogen gas by varying the size of zinc granules which were reacted with dilute hydrochloric acid. All other factors were kept constant in the two experiments. Data obtained were used to plot the following graph: ![]()
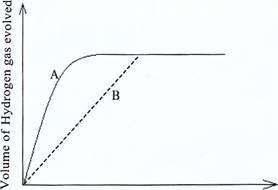
Time
(a) Briefly explain the differences in the results of experiments A and B.
(b) What factors can be adjusted to increase the yield of the product? (7 marks)
View Ans12
(b) The following figure shows the reaction path between sodium hydroxide and hydrochloric acid.
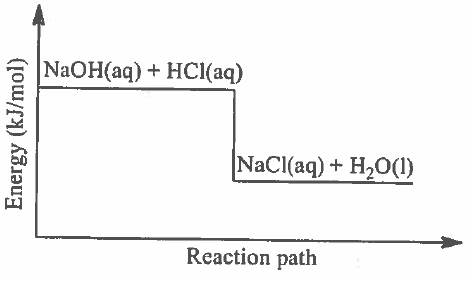
Giving a reason, classify the reaction based on energetics and predict the effects of cooling the system while increasing pressure at the same time. (7 marks)
View AnsHub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






