Two substances are allotropes of carbon if
- Both reduce heated iron (II) oxide to iron
- Have different crystalline structure
- Have equal masses
- Have equal shape
- Have the same arrangement of atoms
2
Which of these can be reduced when heated with carbon?
- Aluminium
- Calcium carbonate
- Iron (III) oxide
- Magnesium oxide
- Sodium oxide.
3
Which of the following sets of elements is arranged in order of increasing electronegativity?
- Chlorine, fluorine, nitrogen, oxygen, carbon
- Fluorine, chlorine, oxygen, nitrogen, carbon
- Carbon, nitrogen, oxygen, chlorine, fluorine
- Nitrogen, oxygen, carbon, fluorine, chlorine
- Fluorine, nitrogen, oxygen, chlorine, carbon.
4
Which of the following pair of gas can be prepared in the laboratory and collected over water?
- Oxygen and Ammonia
- Hydrogen and Hydrochloric acid
- Hydrogen and Ammonia
- Oxygen and Hydrogen chloride
5
Which among the following equations correctly shows the reaction between chlorine gas and water?
- Cl 2(g) + H 2 O (l) ? Cl 2(g)
- 2Cl 2(g) + 2H 2 O (l) ? 4Cl + O 2(g) + 2H 2(g)
- Cl 2(g) + H 2 O (l) ? HCl (aq) + HOCL (aq)
- 2Cl 2(g) + 2H 2 O (l) ? 2HOCl 2(aq) + H 2(aq)
- 2Cl 2(g) + 3H 2 O (l) ? Cl 2(g) + 2H 3 O + .
6
Which among the following pair of substances are allotropes?
- H 2 O and H 2 O 2
- 12 C and 14 C
- P 4 and P 8
- H 2 and 2H +
- H + and H 3 O.
7
In the following equilibrium equation, 2S02(g) +O2(g) ![]() 2S03 The forward reaction is exothermic. Which change would increase the production of sulphur trioxide at equilibrium?
2S03 The forward reaction is exothermic. Which change would increase the production of sulphur trioxide at equilibrium?
- Increasing temperature
- Decreasing temperature
- Decreasing sulphur trioxide concentration
- Decreasing pressure
- Adding a catalyst.
8
The ionic equation when aqueous ammonium chloride reacts with sodium hydroxide solution is represented as:
- 2NH+4(aq) + 2Cl-(aq)à 2NH3(g) + Cl2(g) + H2(g)
- NH+4(aq) + OH-(aq) à NH3(g) + H2O(l)
- Na+(aq) + Cl-(aq) à NaCl(g)
- H+(aq) + OH-(aq) à H2O(l)
- 2NH+4(aq) + 2Cl-(aq) à 2NH3(g) + 2HCl(g).
9
Substance X liberates chlorine gas from acidified potassium chloride. The behaviour of X is described as:
- an oxidising agent
- an oxidising and reducing agent
- catalyst
- a reducing agent
- bleaching agent.
10
In the industrial preparation of sulphur trioxide, equilibrium is established between sulphur dioxide and oxygen gas as follows:
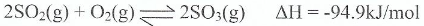
Is the forward reaction an endothermic or exothermic process? Give a reason.
View Ans11
How would you adjust temperature and pressure to maximize the proportion of the product at equilibrium?
View Ans12
Why is it unfavorable to work with very high pressure and very low temperature in the Contact process?
View Ans13
What catalyst is used to speed up the rate of formation of sulphur trioxide before attaining the equilibrium?
View Ans14
Explain each of the following statements and in each give its balanced chemical equation:
- Sulphur dioxide in solution is a powerful reducing agent.
- Sulphur dioxide in solution act as a bleaching agent.
- Sulphur dioxide can reduce chlorine and itself become oxidized.
- When hydrogen sulphide is passed through sulphur dioxide gas, yellow deposits are produced. (7 marks)
15
Match the items in List A with the responses in List B by writing the letter of the correct response beside the item number in the answer booklet provided.Match the items in List A with the responses in List B by writing the letter of the correct
| List A | List B |
|
|
16
The following flow diagram shows the stages in the contact process 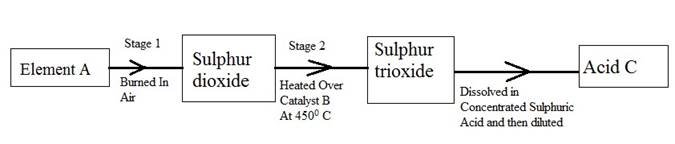
(i) Give the names of element A, catalyst B and an acid C.
(ii) Write a balanced chemical equation for the formation of sulphur trioxide in stage 2
View Ans17
(i) Name the products formed when hydrogen sulphide react with chlorine gas.
(ii) Mention two uses of hydrochloric acid.
View Ans18
Three moles of nitrogen gas combine with five moles of hydrogen gas to form ammonium gas by Haber process.
- Which reactant is present in smaller amount?
- Calculate the grams of the reactant left in the container.
- How many moles of NH3 are produced?
- How many litres of NH3 are produced at STP?
19
Write balanced chemical equations to show how chlorine reacts with the following:
- water.
- aqueous iron (II) chloride solution.
- hydrogen sulphide.
20
The preparation of ammonia in the laboratory is done by heating any ammonium salt with an alkali.
(i) Write a balanced chemical equation for the preparation of ammonia gas.
(ii) State two uses of ammonia.
View Ans21
(i) Explain why sulphur and its compounds are removed from fuels before they are burned.
(ii) Describe how sulphur dioxide is changed into sulphur trioxide. Give the reaction conditions and the equation(s)
View Ans22
(i) Write the reaction equations involved in the industrial manufacturing of sulphuric acid starting with sulphur dioxide in the contact process.
(ii) Explain why sulphur trioxide is not dissolved directly in water to obtain sulphuric acid in contact process.
View Ans23
(a) For each of the following reactions, identify which of the gases, chlorine, sulphur dioxide, and hydrogen sulphide is either an oxidizing agent or reducing agent. Explain how you arrived at your answers.
(i) Cl2(g) + 2H2O(l) + SO2(g) ? 2HCl(g) + H2SO4(aq).
(ii) SO2(g) + 2H2S(g) ? 2H2O(l) + 3S(s).
View Ans24
The formation of oxides of non-metals can be both beneficial and harmful to man. Justify the statement focusing on the oxides of carbon, nitrogen and sulphur.
View Ans25
Match the items in List A with the responses in List B by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
|
|
26
(a) With the help of chemical equation, what will be observed when ammonia reacts with
(i) Hydrogen chloride.
(ii) Copper (II) oxide.
(b) It is not advisable to sleep inside a house which is not well ventilated with a burning wooden charcoal. Give a reason for that and write the chemical equation to represent your answer.
View Ans27
The chemical properties of concentrated sulphuric acid can be grouped into oxidizing property and dehydrating property. In which property should sulphuric acid be grouped when it reacts with copper metal? Give reason and write the equation of the reaction.
View Ans28
Match the items in LIST A with the responses in LIST B by writing the letter of the correct response beside the item number.
| LIST A | LIST B |
| (i) Oxygen (ii) Sulphur dioxide (iii) Ammonia (iv) Hydrogen Chloride (v) Carbon monoxide (vi) Nitrogen (vii) Hydrogen (viii) Chlorine (ix) Nitrogen dioxide (x) Carbon dioxide |
|
29
Ammonia is very soluble in water and less dense than air. How does each of the properties determine the way in which ammonia is collected in a gas jar? (4 marks)
View Ans30
Give reasons for the following:
(i) Solution of chlorine in water is acidic
(ii) Yellow phosphorus is stored under water.
View Ans31
12.(a) Ammonia gas is manufactured by reacting nitrogen gas with hydrogen gas in the presence of a catalyst. Write a balanced chemical equation for the reaction and explain the role played by the catalyst in this reaction.
View Ans32
SECTION C (15 Marks)
Answer one (1) question in this section.
13.Carbon is one of the elements that have allotropes. Explain how the allotropes of carbon differ from each other. (15 marks)
View Ans33
14. Despite its corrosiveness, sulphuric acid is very important in industry. Explain the importance of sulphuric acid in industries by giving six points. (15 marks)
View AnsHub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
WHATSAPP US NOW FOR ANY QUERY
App Ya Learning Hub Tanzania






