



FORM FOUR CHEMISTRY EXAM SERIES 217
FORM FOUR CHEMISTRY EXAM SERIES 217
THE OFFICE OF THE PRESIDENT, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT.
SECONDARY EXAMINATION SERIES
MARCH 2025
CHEMISTRY FORM FOUR
TIME: 2:30HRS
INSTRUCTIONS
- This paper consists of section A, B and C with total of eleven (11) questions
- Answer all questions in section A and B and two (2) question from section C
- Cellular phones and any unauthorised material are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- Non programmable calculator may be used
- The following constant may be used Atomic masse:
- H=1, C=12, N=14, O=16, Na=23, S= 32, Ca=40, Cl= 35.5, Cu= 64 and Zn=65 Avogadro’s number = 6.02 x 1023
- GMV at s.t.p = 22. 4 dm3
- 1 faraday = 96,500 coulombs
- Standard pressure = 760 mm Hg
- Starndard tempreture = 273K
- 1 litre = 1 dm3= 1000cm3
SECTION A (16 Marks)
(Answer all questions in this section)
1. For each of the items (i –x) choose the most from the given alternatives and write its letter beside the item number in the answer sheet (booklet) provided.
(i) When water is added to an acid, the acid becomes
- More acidic and its pH goes down
- More acidic and its pH goes up
- Less acidic and its pH goes down
- Less acidic and its pH goes up
- Neutral and its pH become 7
(ii) 1.4g of potassium hydroxide is dissolved in water to form 250cm3 of Solution.
What is the Molarity of this solution?
- 0.001M
- 0.1M
- 1.4M
- 5.6M
- 6.0M
(iii) An electric current was passed through a concentrated solution of hydrochloric acid using carbon electrodes. The substance liberated at anode was.
- Copper
- Hydrogen
- Oxygen
- Sodium
- Chlorine
(iv) If element Q of group (H) combines with element R of group (IV) what will be the formula of the resulting compound.
- R2Q
- QR6
- R3Q
- R3Q
- Q2R
(v) The IUPAC name of H2SO4 is:
- Sulphuric (IV) acid
- Sulphuric acid
- Sulphuric (V) acid
- Sulphorous acid
- Sulphuric (VI) acid
(vi) Which of the following are the components needed to start fire?
- Match box, fire wood and kerosene
- Match box, fire wood and oxygen
- Oxygen, fuel and heat
- Oxygen, fuel and fire wood
- Heat, match box and oxygen
(vii) Which of the following is NOT a component of the first aid kit?
- Goggles
- A pair of scissors
- Dropper
- Gloves
- Razor blade
(viii) Which among of the following chemical reactions rapidly releases energy in form of light and heat?
- Combustion
- Decomposition
- Displacement
- Neutralization
- Precipitation
(ix) Laboratory Technician prepared a solution containing 26.5g of anhydrous Sodium carbonate in 5 dm3 of the solution and provided to Form Four students to calculate Its Molarity. Which among the following will be the possible answer?
- 0.05
- 0.25
- 1.25
- 5.3
- 0.025
(x) The following reaction 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O (l) is an example of a
- Redox reaction
- Combination reaction
- Esterification
- Neutralization reaction
- Decomposition reaction.
2. Match the item in LIST” A” with the correct response in LIST” B”
| LIST A | LIST B |
| (i) Methyl orange indicator (ii) Calcium hydroxide (iii) pH 2 (iv) Neutralization reaction (v) Sodium hydrogen sulphate |
|
SECTION B (70 MARKS)
3. (a) Write balanced equation of
(i) Sodium hydroxide react with sulphuric acid
(ii) Calcium carbonate decomposed by heat
(b) (i) With aid of a balanced chemical equation name the products formed when nitrates of potassium and zinc decompose by heat
(ii) Suggest why nitrates of zinc and potassium behave differently on heating
4. (a) (i) People suffering from heart burn usually use wood ashes for relief. Mention chacteritics which makes the ashes to be used for heart burn relief.
(ii) Give four compounds found in the laboratory which show the same characteristic as ashes.
(b) How many ions are there in 6.82g of Al2(SO4)3
5. (a) When an acid is reacted with base, it forms salt and water. Using your knowledge of chemistry, explain how will you apply this reaction in your daily life? Give any four points.
(b) Insoluble salts are the salt that does not dissolve in water. Name any three examples of salts that are insoluble in water. (07 Marks)
6. 16.8g of impure potassium hydroxide was dissolved in distilled water to make 1000mls. 20mls of this solution required 28mls of 0.07M sulphuric acid to react. Calculate:
(a) Molarity of KOH
(b) Mass Concentration of pure KOH (07 Marks)
7. a) Mr Mwakatumbula don’t understand the physical properties of water. Teach him by giving three points the main physical properties of water and show the usefulness of each property.
b) Mr Kadinya said that Electrolysis is applied by many areas on the earth. State four industrial application of electrolysis
8. (a) Study the following reaction equation N2(g) + 3H2(g)![]() 2NH3(g) ∆H = - 46.2 kJmol-1
2NH3(g) ∆H = - 46.2 kJmol-1
Use Le-Chatelier’s principle, suggest how you would use temperature and pressure to obtain the highest production of ammonia at equilibrium
(b) The formation of methanol from hydrogen and carbon monoxide can be represented by
CO(g) + 2H2(g) ![]() CH3OH ∆H = 91 kJmol-1
CH3OH ∆H = 91 kJmol-1
What mass of hydrogen would react to cause a heat change of 91 kJ
9. (a) Give the meaning of the following terms
(i) Soil pH (ii) Liming
(b) (i) Explain why sulphur and its compounds are removed from the fuel before they are burned
(ii) By using a reaction equation explain how propane differs from propene
10. (a) In electrolytic production of hydrogen gas, dilute mineral acid is used. Which Method is used in its collection? Give a reason.
(b)Explain the chemical preference of decorating a copper necklace with silver metal by using electrolysis method
(c)During electrolysis of brine, sodium was deposited at cathode and chlorine gas released at anode. If 2.0g of sodium were collected at cathode; find the volume of chlorine gas at s.t.p.
SECTION C.
ANSWER QUESTION 11
11. a)Metals are extracted from the sea and in earth Referring to Tanzania as among the countries in the world extracting metals, explain four stages of extraction of metals.
b). Does the extraction of gold follow all four stages? Give reasons.
FORM FOUR CHEMISTRY EXAM SERIES 204
FORM FOUR CHEMISTRY EXAM SERIES 204
PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM FOUR
MID-TERM EXAMS – AUG/SEPT – 2024
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of eleven (11) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A
Answer all questions in this section
- For each of the items (i) - (x) choose the correct answer from the given alternatives and its letter in the answer sheet provided.
- Zinc reacts with dilute acids displacing hydrogen, but copper does not. This indicates that; -
- Zinc is higher in the activity series than hydrogen and copper
- Zinc is less reactive than copper
- Copper is more electropositive than zinc
- Copper is inert
- Copper is found above zinc and hydrogen in the electrochemical series.
- The reason that made scientists to stop using hydrogen gas in filling balloons is; -
- The gas became denser than air
- The gas is less dense than air
- Discovery of helium gas
- The gas is soluble in water
- The gas is highly flammable and burns with pop sound.
- What fire extinguisher will you use to help your friend whose shirt caught fire during an experiment in the laboratory?
- Powder fire extinguisher
- Fire blanket
- Carbon dioxide
- Water
- Foam.
- The nuclide notation of element Z is 1531Z which set of sub-atomic particles is collect?
- 15 protons, electrons 16, and 15 neutrons
- 15 protons,15 electrons, and 16 neutrons
- 15 protons, 15 electrons and 15 neutrons
- 16 protons, 16 electrons and 15 neutrons
- 16 protons, 15 electrons, and 15 neutrons
- A form four student was given the following staffs for preparation of ammonia gas.
- Source of heat
- Calcium hydroxide
- Ammonium chloride
- Solid potassium hydroxide
- A rapid chemical reaction that release energy in form of light and heat is called; -
- Neutralization
- Decomposition
- Combustion
- Precipitation
- Displacement
- Which of the following pairs constitute the best methods for treating and purifying water?
- Chlorination and distillation
- Chlorination and sedimentation
- Disinfection and decantation
- Disinfection and filtration
- Chlorination and aeration
- The equation below shows the dissociation of sulphuric acid into ions: -
![]()
![]() H2SO4(aq) H+ (aq) + SO42- (aq). From the equation, how many ions are there in 9.8g of H2SO4?
H2SO4(aq) H+ (aq) + SO42- (aq). From the equation, how many ions are there in 9.8g of H2SO4?
- 2.9x1023 ions
- 1.22x 1023ions
- 8.9 x1023ions
- 1.81x1023ions
- 0.3x1023ions
- Why oxygen differs from other gases?
- It explodes and support combustion
- It neither burns nor support combustion
- It burns but does not support combustion
- It supports combustion but does not burn
- It burns and support combustion
- In the following equilibrium equation
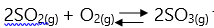
the forward reaction is exothermic. Which change would increase the production of Sulphur trioxide at equilibrium?
- Adding a catalyst
- Decreasing pressure
- Increasing temperature
- Decreasing Sulphur trioxide concentration
- Decreasing temperature
- Match the items in list A with the correct response in List B by writing the letter of the correct response in the box provided bellow
| LISTA | LISTB |
|
|
SECTION B (54 marks)
Answer all questions in this section
- (a) Form four students wanted to verify the presences of water in an unknown compound using hydrated copper (II) sulphate. A small amount of hydrated copper (II) sulphate was placed on watch glass followed by addition of few drops of the unknown compound. Then was no change in the colour observed.
- Why was there was no change in the colour of hydrated copper (II) sulphate?
- What other substance that could be in the place of the hydrated copper (II) sulphate to observe the required colour change.
(b) What is so called the universal solvent comment on this statement
4. Chemistry teacher guided students of form four to study carefully an experiment carried out in order to distinguish various substances formed during an experiment. The procedures were as follows
- Measure about 120cm3 of water and pour it in a beaker
- Add a spatula full of common salts and stir to allow salts to dissolve
- Continue to add more salts to the solution, stirring until no more salts can dissolve
- Place the solution on tripod stand and heat gently as you continue stirring
- Stop heating when salts dissolves
- Place the beaker in a trough of cold water and allow cooling for 3 to minutes note the results observed
- What type of solution was obtained when a spatula full of table salt were dissolved in 120 cm3 of water at room temperature? Give reasons
- What type of solution was obtained when no more salts dissolved in cold water at room temperature? Give reason
- What is the name of the final solution obtained after cooling through of coldwater? Give reason
- Explain the two (2) application of the concept studied above
5. Mwasiti was supplied with two beakers, one containing 0.1M KOH standard solution, and the other beaker containing sulphuric acid solution whose molarity was not known. She was asked to find the molarity of the acidic solution. Mwasiti took the basic solution and put it into the burette, then she measured accurately 25cm3 of sulphuric acid using a measuring cylinder and transferred it into a conical flask, she is then added few drops of methyl orange indicator.
Her titration results were showing that 25cm3 of acid required 2cm3 of KOH standard solution.
She used the following formula to calculate the molarity of the sulphuric acid.
Molarity of H2SO4 acid = (Volume of acid x Volume of base)/Molarity of base
(a) What are the mistakes Mwasiti performed in her titration? (Four mistake).
(b) For each mistake, suggest the correct measure you would take.
6. (a) A person suffering from in digest ion produces1.0Litre of gastric juices per day which contains about 2.0g of hydrochloric acid. How many antacid tablets each containing 400mmg of sodium bicarbonate (NaHco3) is needed to neutralize all the hydrochloric acid produced in a day?
- Name the suitable warning sign that you would find on the following: -
- A bottle containing concentrated sulphur in a day?
- A bag contain ingrator insect poison
- Room door containing x–ray machine
- Bag containing dynamites
7. Explain how the following factors affect the rate of chemical reaction
- Pressure (ii) Temperature
- Sodium hydroxide was dissolved in water after a while the container. Holding the solution was observed to be hat
- Basing on the heat change, name there action that took place in the above process
- Suggest any two application of there action above in our everyday
- Draw energy level diagram for the above process named in b(i)
8. During electrolysis of an aqueous of salt of metal X, a current of 2.0A was passed for 26 minutes and 32 seconds. The mass of metal ‘X’ deposited was 0.24g.
- On which electrode was the metal X deposited?
- Calculate the amount of charge needed to deposit 1 mole of metal X.
- Calculate the charge carried on the ion.
- Write an ionic equation to show how the ions of the metal X are discharged at the electrode (R.A.M. of metal X= 24)
SECTIONC (30 MARKS)
Answeronlytwo(02) questionsfromthissection.
9. Agriculture is back bone of our economy but the addition of in organic fertilizers in the farm is not as important as addition of organic manure. Discuss the correctness of this statement in four points.
10. The extraction of metals has economic advantageous in Tanzania, but it’s still destructive to the environment. Illustrate three (3) environmental destructions may be caused by the process, and suggest three (3) control measure to problem.
11. (a)Name the process used to obtain an aqueous solution of ethanol from a sugar, for example Glucose (C6H12O6)
(b) States without further description, the method you would use to obtain a sample of reasonably pure ethanol from the aqueous solution, and indicate the physical properties to which this separation in based.
(c) Briefly explain the following chemical reaction which takes place when: -
- Ethanol burns in air with a pale blue flame.
- Sodium is added to a pure ethanol; a colorless gas is evolved.
FORM FOUR CHEMISTRY EXAM SERIES 192
FORM FOUR CHEMISTRY EXAM SERIES 192
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
PRE- MOCK CHEMISTRY FORM FOUR
Time: 3Hours
Instructions
- This paper consists of sections A, B, and C with a total eleven (11) questions.
- Answer all question in the sections A, B and two (2) questions from section C.
- Section A carries sixteen (16) marks, section B fifty four (54) marks and section C carries thirty (30) marks.
- All writing should be in blue or black pen, except for diagrams that must be drawn in pencil.
- Communication devices and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet (s)
SECTION A.
- For each of the following items (i-x).Choose the correct answer from given alternatives and writer its letter besides the item number in the answer booklet
- Which type of bonding is expected in a compound formed between potassium (K) and chlorine (Cl)?
- Ionic
- Covalent
- Metallic
- Hydrogen Bonding
- Dipole-Dipole Interactions
- The mass number of an atom represents the total number of:
- Protons only
- Neutrons only
- Electrons only
- Protons and neutrons
- Protons and electrons
- How many moles of carbon dioxide (CO2) are present in 88 grams of CO2?
- 0.5 moles
- 1 mole
- 2 moles
- 4 moles
- 88 moles
- When heating a flammable liquid in a laboratory, which of the following is the safest practice?
- Direct heating with a Bunsen burner
- Heating in a water bath
- Heating in an open test tube
- Adding flammable liquids to an open flame
- Leaving the experiment unattended
- Which separation technique would be most appropriate for separating a mixture of water and ethanol?
- Filtration
- Chromatography
- Simple distillation
- Fractional distillation
- Evaporation
- During the electrolysis of molten sodium chloride (NaCl), which of the following substances is formed at the cathode?
- Chlorine gas (Cl2)
- Sodium metal (Na)
- Hydrogen gas (H2)
- Oxygen gas (O2)
- Hydrochloric acid (HCl)
- For a reversible reaction, a large value of the equilibrium constant (Kc) indicates that:
- The reaction proceeds very slowly
- The forward reaction is favored
- The reverse reaction is favored
- The reaction is at equilibrium
- The reaction does not take place
- Which metal is commonly extracted from its oxide ore using electrolysis?
- Iron (Fe)
- Gold (Au)
- Sodium (Na)
- Copper (Cu)
- Zinc (Zn)
- Chlorine reacts with sodium hydroxide (NaOH) to form a common household disinfectant. What is the main active ingredient in this disinfectant?
- Hydrochloric acid (HCl)
- Sodium chlorate (NaClO3)
- Sodium hypochlorite (NaClO)
- Sodium chloride (NaCl)
- Sodium chlorite (NaClO2)
- Which type of reaction is most characteristic of saturated hydrocarbons?
- Substitution
- Addition
- Polymerization
- Condensation
- Combustion
2. Match the compound in LIST A with the correct way to identify it in LIST B and write the letter of the correct answer on sheet provided.
| LIST A | LIST B |
|
|
SECTION B: 54 Marks
3(a) State two reasons why we use the non-luminous flame for heating in a laboratory instead of using the luminous flame.
(b) Chlorine has two isotopes with atomic mass 35 and X occurring in the ratio 3:1 respectively. The relative atomic (R.M.A) of chlorine is 35.5. Determine the value of X.
(c) In an experiment to electroplate iron with silver, current of 1 Ampere was passed through a silver solution of ions for 60 minutes.
(i) Give a reason why it is necessary to electroplate iron.
(ii) Calculate the mass of silver deposited on iron during the electroplating process. (Ag = 108, IF = 96500c)
4. (a) Calculate the volume of 0.6M sulphuric (VI) acid solution needed to neutralize 30cm3 of 0.2Mpotassium hydroxide.
(b) A state of equilibrium between dichromate (vi) and chromate ions is established as shown below
Cr?O?²? (aq) + H?O (l) ? 2 CrO?²? (aq) + 2 H? (aq)
Orange (Yellow)
i. What is meant by dynamic equilibrium?
ii. State and explain observation made, when a few pellets of Potassium Hydroxide are added to equilibrium mixture
(c ) The following reaction takes place in a closed system:
N?(g) + 3H?(g) ? 2NH?(g) ΔH = -92 kJ/mol
Consider the following scenarios:
Scenario 1: More nitrogen (N?) gas is added to the system.
Scenario 2: The temperature of the system is decreased.
Scenario 3 A catalysts is added to the system.
For each scenario:
a) Predict the direction in which the equilibrium will shift (left, right, or no change). b) Explain your reasoning using the principles of chemical equilibrium and Le Chatelier's principle.
5. Use the diagram below to answer the questions that follow.
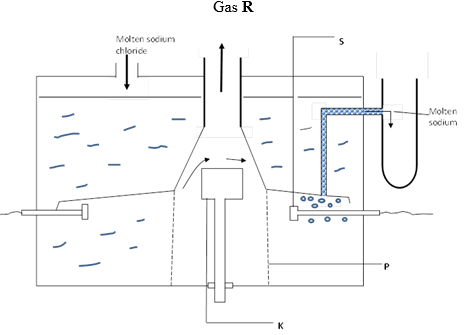
(a) Identify the substances labelled R, S, K and P (b) What is the function of the part labelled P?
(c) Write half equations at the electrodes.
(d) Why is molten sodium chloride used instead of sodium chloride solution?
(e) Why is calcium chloride added in the electrolysis of molten sodium chloride?
(f) How is the calcium eventually separated from the sodium?
(g) When sodium is left exposed in the air a white solid is formed but when sodium is burnt in oxygen, a yellow solid is formed. Explain this difference using equations.
6. Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
| Element | Atomic number | Melting point 0C |
| R | 11 | 97.8 |
| S | 12 | 650.0 |
| T | 15 | 44.0 |
| U | 17 | -102.0 |
| V | 18 | -189.0 |
| W | 19 | 64.0 |
(a) Give a reason why the melting point of;
(i) S is higher than that of R.
(ii) V is lower than that of U. (b) How does the reactivity of W with chlorine compare with that of R with chlorine?
(c) When 0.30g or R was reacted with water 1600cm3 of gas was produced. Determine the relative atomic mass of R. (Molar gas volume = 24000cm3 r.t.p
(d) Give one use of element V.
(e) Draw a structure of the compound formed when S reacts with U.
(f) Compare the atomic radius of element S and V. Give a reason
7. (a) Hard water has both advantages and disadvantages. Give one advantage and one disadvantage of using hard water
(b) Using an equation, explain how addition of sodium carbonate is used to remove water hardness.
(c) Outline three importance of a chemical equation.
8. I. In an experiment, copper metal was heated in the air to form a black solid T. dilute Sulphuric (VI) acid was then added to solid T resulting to formation of solution W, after which Ammonia was then added to solution W drop wise till excess
(a) Identify solid T
(b)Write a chemical equation for the reaction leading to formation of solution W
(c) State the observations made when the ammonia solution was added to solution W dropwise till excess.
II. Substance A is a solid that does not conduct electricity at room temperature. However, when molten, it becomes a good electrical conductor.
Substance B is a solid with a high melting point and can conduct electricity in the solid state.
a) Suggest the likely types of bonding present in Substance A and Substance B.
b) Explain the differences in their electrical conductivity in both solid and molten/liquid states.
(c) An experiment is set up to electrolyze a concentrated solution of sodium bromide (NaBr).
i) Identify the products that would form at the cathode and anode.
ii) Explain your reasoning, including relevant half-equations.
iii) Describe any observable changes expected during the electrolysis process.
SECTION B: 30 MARKS
Answer any two questions
9.(a) What name is given to each of the following?
(i) Ability of a metal to be beaten/ hammered to a sheet
(ii)Force of attraction that holds two molecules together
(b) When 3.1g of Copper {II} Carbonate were heated in a crucible until no further change in mass, solid L and gas M were formed
(i) Identify solid L and gas M
(ii) Write a chemical equation for the reaction that occurred
(iii) Given Cu=64, C=12,O=16, calculate the mass of the solid L that was formed
10 (a) Give the name of the following processes.
(i) A hot saturated solution of copper (II) sulphate is cooled to form crystals of copper (II) sulphate.
(ii)A white powder is formed when concentrated sulphuric (V) acid is added to blue hydrated copper
(II) sulphate.
(b)Study the flow chart below and answer the questions that follow.
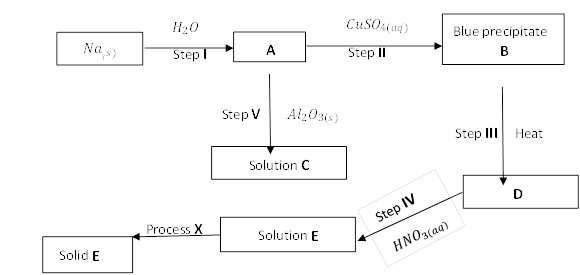
(i) Name substances: B, C, D, and Solid E
(ii) Write equations for the reactions in steps; III and V
(iii) Write the ionic equation for the reaction in step II.
(iv) State any two observations made in step I.
11. (a) Addition of inorganic fertilizers in the farm is not as important as addition of organic manure. Discuss the correctness of this statement in four (4) points
(b) Soil fertility and soil productivity are mistakenly used to mean the same concept. How do they differ from each other? Give five points
FORM FOUR CHEMISTRY EXAM SERIES 185
FORM FOUR CHEMISTRY EXAM SERIES 185
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
MID TERM ONE – MARCH-2024
CHEMISTRY FORM FOUR
Time: 3Hours
Instructions
- This paper consists of sections A, B, and C with a total eleven (11) questions.
- Answer all question in the sections A, B and two (2) questions from section C.
- Section A carries sixteen (16) marks, section B fifty four (54) marks and section C carries thirty (30) marks.
- All writing should be in blue or black pen, except for diagrams that must be drawn in pencil.
- Communication devices and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet (s
SECTION A.
Answer all questions in this section.
- For each of the following items (i-x).Choose the correct answer from given alternatives and writer its letter besides the item number in the answer booklet provided
- An increase in temperature of a gas in enclosed system container caused an increase pressure of the gas. This is because it increases the ;
- Number of gas molecule
- Combination of gas molecule
- Number of collision between gas molecules
- Average velocity of gas molecules
- Kinetic energy of gas particles
- 1.4g of potassium hydroxide is dissolved in water to form 250cm3 of solution. What is the molarity of the solution
- 0.001M
- 0.1M
- 1.4M
- 5.6M
- 6.0M
- When methane undergoes substitution reaction with excess chlorine, What is the final product?
- Chloromethane
- Dichloromethane
- Tetrachloroethane
- Tetrachloromethane
- Monochloromethane
- Ethanol reaction with ethanoic acid to form a group of organic compound called
- Alkynes
- Halo-alkanes
- Esters
- Alkenes
- Alkanes
- Insoluble salts like Barium Sulphate generally can be obtained in laboratory by;
- Evaporation of its concentrated solution
- Crystallization
- Precipitation
- Decomposition
- Displacement
- In a blast fumace carbon monoxide is prepared by passing carbon dioxide over red hot coke. What is the chemical role of carbon dioxide
- An acceleration
- An oxidizing agent
- Reducing agent
- A catalyst
- Oxidized
- What is the oxidation number of Phosphorus in the following compound? H3PO4
- -5
- 0
- +2
- +5
- -3
- Which of the following is not an organic compound?
- CO
- C6H12O6
- CH4
- CH3COOH
- C2H5B1
- A metal Nitrate which will not give a metal oxide on heating is
- Calcium Nitrate
- Silver Nitrate
- Lead Nitrate
- Copper Nitrate
- Zink Nitrate
- What action should be taken immediately after concentrated Sulphuric acid is spilled on the skin
- It should be rinsed off with large amount of water
- It should be neutralized using concentrated NaOH
- The affected area should be wrapped tightly and shown to a medical health provider
- It should be neutralized using solid CaCO3
- It should be neutralized with concentrated KOH
- Match sources of energy in the list A with corresponding description in list B. Write letter of the correct answer beside item number
| LIST A | LIST B |
|
|
SECTION B
54 MARKS
Answer all questions
- (a) Study the following reaction equation N2(g)+3H2(g) H = -46.2KJlmo1-1
Use le chateliers Principle, suggest how you would use temperature and pressure to obtain the highest production of ammonium in equilibrium
(b) The formation of methane from hydrogen and carbon monoxide can be represented by:
CO(g) +2H2g CH3OH DH= 91KJ.........
What mass of hydrogen would cause heat change of 91KJ?
- What is the importance of having fume chamber in Chemistry Laboratory
(ii) Why do laboratory doors open outwards?
(b) State the use of following item in first aid
- Cotton wool
- Petroleum jelly
- Pain killers
- Bandage
- Razor blade
- Define the following terms;
- Molecular formula
- Empirical formula
(b) You are provided with compound 22.2%Zuric, 11.6% Sulphur, and 22.3%. Oxygen and the rest water of Crystallization. Calculate the molecular formula of the compound if its molecular mass is 283.
- 16.8g of impure potassium hydroxide was dissolved in distilled water to make 1000mls. 20mls of this solution required 28mls of 0.07M saulphuric acid to react. Calculate :
(a) molarity of KOH
(b) Mass concentration of pure KOH
- (a) The modern periodic law is based modification of Mendeleev periodic law. Explain how the two theories differ from each other.
(b) Comment on the following statement
(i) Lithium has large size than beryllium
(ii) Sodium is smaller than Potassium
(c) gives any four ions whose electronic configuration resemble that of Neon.
- (a) state faradays maw of electrolysis
Using the law state above, derive mathematical expression of Faradays first law of electrolysis.
(b) How many moles of electrons will be transferred is Zinc metal is produced by a current of 14.23A. Supplied for 8.0hrs.
SECTION C
30 MARKS
Answer 2 questions from this section.
- With aid of balanced chemical equation; explain the process that occurs in blast fumace during extraction of irons.
- (a) define the following terms
- Functional group
- Homologous series
- Isomerism
(b) Write down the molecular structure and IUPAC names of the Isomer whose molecular structure is C4H10
(c) By naming reagent, stating conditions whenever possible using a balanced equation describe how Ethane could be converted into
- Ethane
- Chloromethane
- 1,2,- dibromoethane
- Dilute Nitric acid is added to a green solid P.A blue solution R is formed and gas I Precipitate Lime with lime water is formed, the blue solution R is evaporated to dryness in a pyres test tube to give black solid M, brown fumes of gas W and colorless gas which relights a glowing splint was formed.
(a) Identify Substance P, R, I, M, W, and S
(i) Dilute nitric acid and solid P
(ii) Formation of white precipitate with gas I and Lime water.
FORM FOUR CHEMISTRY EXAM SERIES 177
FORM FOUR CHEMISTRY EXAM SERIES 177
PRESENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM FOUR
MID-TERM EXAMS – AUGUST – 2023
032/1 CHEMISTRY 1
TIME: 3:00 Hours YEAR. 2023
Instructions
- This paper consists of sections A, B and C with a total of eleven (11) questions.
- Answer all questions in section A and B and any two (2) question from section C
- Section A carries sixteen (16) marks, section B carries fifty four (54) marks and section C carries thirty (30) marks.
- All writing must be in blue or black ink except drawings which must be in pencil
- All communication devices and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer sheet(s) provided.
- The following constants may be used;
- Atomic masses H=1, C=12, K=39, O=16,S=32, Cl=35.5
- Avogadro’s constant = 6.02x1023
- GMV at S.T.P = 22.4dm3
- 1Faraday =96500C
- 1Litre=1dm3=1000cm3
SECTION A: (16 Marks)
Answer all questions
- For each of the following items (i) – (x) choose the correct answer among the given alternatives and write its letter besides the item number on the answer sheets provided.
- A form one student saw the "flammable" sign on a box and made the following possible interpretations. Which one is the most correct?
- The box contained firewood
- The box contained papers
- The box had radioactive materials
- The box contained spirit used in lamps
- The box contained health hazards
- What should be done if the results obtained from an experiment do not support the hypothesis?
- Experiment should be changed
- Results should be left out
- Ideas for further testing to find a solution should be given
- Problem should be identified
- Hypothesis should be accepted
- The reaction between potassium metal and water is
![]()
Which of the following statement is true about this reaction?
- Hydrogen is oxidized
- potassium is reduced
- Potassium is the reducing agent
- Hydrogen is the reducing agent
- Water is both oxidizing and reducing agent
- How many molecules are there in 16.8litres of chlorine gas at STP?
- 4.517 X1023
- 7.5 X1023
- 8.029 X1023
- 9.033X1023
- 6.75 X1023
- Ammonia is the basic gas that can obtained by reacting ammonium salt and calcium hydroxide. Which of the following can be used to dry ammonia during its preparation.
- Concentrated sulphuric acid.
- Calcium Chloride
- Lime water
- Calcium oxide.
- Calcium Carbonate
- Mr John treated water for domestic purpose, unfortunately the water remained hard on boiling and formed no leather with soap. Suggest the ions present in the water.
- Ca2+ and HCO3-.
- Ca2+ and CO3 2-
- Mg2+ and SO32-
- Mg2+ and HCO3-.
- Mg2+ and SO42-.
- A mixture of 100cm3 of ethanol and 100cm3 of pure water can be separated by....
- Solvent extraction
- Simple distillation
- Evaporation
- Fractional distillation
- Filtration
- If Mr. Kashamba wants to electroplate his spoon with copper sulfate solution, he should arrange the electrode in the following way...
- Spoon as Anode and copper cathode.
- Spoon cathode and copper Anode
- Spoon Anode and carbon cathode.
- Spoon cathode and copper(II) sulphate anode
- Carbon cathode and copper Anode
- Which of the following sets represents isotopes of an element?
- 167K , 78K and 189K
- 167K , 169K and 168K
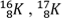 and
and 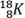
- 168K , 178K and 189K
- 169K , 168K and 178K
- Asha harvest unripe mangoes from her fruit garden, she then keep them till ripe and sell the mangoes. What changes occur for mangoes to turn ripe from unripe?
- Physical change.
- Chemical change.
- Physical and chemical change
- Neither physical nor chemical change.
- Physical change and taste change
2. Match the uses of apparatus in list A with the respective element in list B by writing the letter of the correct response besides the item number in the answer sheet provided
| LIST A | LIST B |
| i. Holding , heating and estimating the volume of liquids ii. Holding substances that are being weighed or observed iii. Holding chemicals and heating small portions of chemicals of liquid or solid form iv. Adding small volume of liquids reagents to an exact point during experiment v. Holding and measuring liquid during experiment vi. Measuring mass of chemicals in the laboratory |
|
SECTION B (54 Marks)
Answer all questions in this section
4, Juma was given two gas jars, one containing gas “X” and another one containing gas “Z” Gas “X” is used to prepare water gas in the laboratory and gas “Z” is used treating sewage plants
- Identify the two gases
- With reasons give the method used to collect gas “Z” in the laboratory
- Give three uses of gas “X” in our daily life
4. 16.8 g of impure potassium hydroxide was dissolved in distilled water to make 100 ml. 20mls of this solution required 28mls of 0.07M sulphuric acid to react. Calculate;
- Molarity of KOH
- Mass concentration of pure KOH
5. (a) A form three student performed two different experiments
Experiment I; Students dissolved one tea spoon of sugar in cold water
Experiment II: Students dissolved one tea spoon of sugar in hot water
By using the concept of collision theory explain in which experiment the sugar dissolved more quickly?
(b)You are provided with the following equilibrium reaction
![]()
What happens to the production of ammonia if
- Pressure increased
- The equilibrium system is cooled
- Hydrogen is removed
- Nitrogen increased
6. Samwel bought all necessary building materials as he wanted to build modern and expensive house for his mother, four months later he found that almost all nails and iron sheets have been rusted, unfortunately he has no any extra money to buy the new nails and iron sheets. As a chemist student what will you advise Samwel (Give two points)
(b) Suppose you are in a bus travelling for annual holiday, a bus driver went to the petrol station to fill fuel but he forget to switch off the bus engine, this caused explosion hence the fire started.
As a form four student
- How will you be able to use fire extinguisher to fight against fire?
- Which type of fire extinguisher will be suitable for you to use? Give reason of your choice
7. (a)What are the four stages for extraction of moderate reactive metals
(b) In certain areas iron can be extracted through blast furnace which involve different temperatures such as10000C, 7500C and 2500C in different stages
- What is the chief ore in the extraction of iron
- At what temperature reduction taking place?
- What are the importance of coke, hot air and waste gases
(c).What are the two environmental effects caused by extraction of metals
8. During manufacturing of Sulphuric acid, Sulphur trioxide gas is dissolved in concentrated sulphuric acid instead of dissolving it directly in water
- Explain why? And Write a balanced equation of dissolution of the gas in the acid
- Write a balanced chemical equation for each of the following
- Sulphuric acid act as an acid
- Sulphuric acid act as an oxidizing agent.
SECTION C (30 Marks)
Answer only two questions from this section
9. Explain the term substitution reaction as applied in organic chemistry
(b)With the use of chemical tests differentiate between saturated hydrocarbons and unsaturated hydrocarbons.
(c). Write and name all isomers of pentane
10. The head master of Mtakuja secondary school advised an environmental master to use manure and fertilizers in order to improve the growth of trees and plant around the school .What are the significance of manure and fertilizers as per head masters advise.(Five points)
11. There are various environmental plans to be used in controlling the solid waste and soil pollution in Mbozi municipality. Advise accordingly to the effective plans to be taken in order to achieve such activities.( Five points)
FORM FOUR CHEMISTRY EXAM SERIES 163
FORM FOUR CHEMISTRY EXAM SERIES 163
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM FOUR
TERMINAL EXAMS MAY – 2023
032/1
CHEMISTRY
TIME: 3HOURS
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of eleven (11) questions.
- Answer all questions in sections A and B and two (2) questions from section C.
- Non-programmable calculators may be allowed.
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer sheet(s)
- The following constants may be used: Atomic masses:
Atomic masses; H=1, O=16, Na=23, C=12, Cl=35.5, N=14, Zn=65, S=32,
Ca=40, Fe=56, Cu=64, Al=27
GMV at s.t.p = 22.4dm3, Avogadro’s number = 6.02 x 1023
1Faraday = 96500 coulomb 1 litre = 1dm3=1000cm3
SECTION (16 MARKS)
- For each of the items (i – x) choose the correct from the given alternatives and write it’s letter in the answer sheet provided
- A substance which absorb water from the atmosphere and form a solution is called ....
- Efflorescent
- Hygroscopic
- Deliquescent
- Amphoteric
- Technicians prefer to use blue flame in welding because
- It is bright and non soot
- It is light and non soot
- It is very hot and non soot
- It is not expensive
- Chlorine ion Cl- differ from chlorine atom because it has
- More proton
- Less proton
- More electron
- Less electrons
This is a good example of
- Neutralization reaction
- Double decomposition reaction
- Redox reaction
- Synthesis reaction
- The volume of 0.2M of H2SO4 acid required to neutralize completely 25.00cm3 of 0.05MKOH is...........
- 0.626cm3
- 3.125cm3
- 12.500cm3
- 6.315cm3
- An electric current was passed through concentrated hydrochloric acid using carbon electrodes. The substance liberated at the anode was
- Copper
- Chlorine
- Oxygen
- Hydrogen
- A good charcoal burns with................
- Luminous flame
- Non-luminous flame
- Very low energy value
- High production of gases
- A covalent bond is formed when
- Ammonia is formed
- Potassium and Oxygen combine
- A metal combines with nonmetal
- An atom loses an electron.
- The empirical formula of a certain compound is CH3. Its molar mass is 30g/mol. What will be its molecular formula?
- C2H6
- CH4
- C2H8
- C2H4
- ........ is the general terms used to explain a mixture of different metals
- Amphoteric
- Allotrope
- Isotope
- Alloy
- Match the item in LIST A with the correct response in LIST B
| LIST A | LIST B |
|
|
SECTION B (54 MARKS)
Answer all questions from this section
- (a)Write balanced equation of
- Sodium hydroxide react with Sulphuric acid
- Calcium carbonate decomposed by heat
(b)(i)Wit aid of a balanced chemical equation name the products formed when nitrates of potassium and zinc decompose by heat
(ii)Suggest why nitrates of zinc and potassium behave differently on heating
- (a)(i)People suffering from heart burn usually use wood ashes for relief.
Mention characteristics which make the ashes to be used for heart burn relief
(ii)Give four compounds founds in the laboratory which show the same characteristic as ashes.
(b)How many ions are there in 6.82g of Al2(SO4)3
- A student tested four sample of water each 10cm3 from different areas at Malawi village by shaking by shaking with tree drops of soap solution. The experiment was repeated a second time by boiling each sample of water (10cm3) with three drops of soap solution. The observations were recorded as shown in the table below.
| Sample | Observation with soap solution | Observation for boiled sample with soap |
| | No lather | Lather |
| | Lather | Lather |
| | Lather | Lather |
| | No lather | No lather |
- Which sample contains only temporary hard water? Give reason
- Which sample contains permanent hard water? Give reason
- Name the chemical substances that would be the causes of hardness in sample A
- Write the chemical equation for removing hardness in
- Sample A
- Sample D
- 5.3g of X2CO3 was dissolved in water to make 0.5 litres of a solution. 25cm3 of this solution required 50.0cm3 of 0.1MHCL for complete neutralization.
- Write the balanced chemical equation for the reaction
- Calculate the concentration of X2CO3 in mol/dm3
- Calculate the relative molecular mass of X2CO3
- Calculate the relative atomic mass of X
- What is the name and symbol of element X
- (a)Give two example in each of the following
- Solid fuel
- Gaseous fuel
(b)The reaction which produces methanol from carbon monoxide and hydrogen is represented by the equation
The reaction is carried out at high pressure to give a better yield of methanol.
- Explain why increase in pressure gives a better yield of methanol
- The value of
is negative. What does this tell about the reaction
- With reason state whether a high temperature or low temperature will give a better yield of methanol
- Draw the well labeled diagram of laboratory preparation of Oxygen using the mixture of Potassium Chlorate and Manganese (iv) Oxide.
SECTION C (30 MARKS)
Answer only two (2) questions from this section,
- With the aid of the balanced chemical equations explain the processes that occur in the blast furnace during extraction of iron.
- (a)Briefly explain the following observations with the help of equations
- White anhydrous copper(II) sulphate changes its colour to blue when water is added
- Vigorous reaction takes place when a small piece of sodium is placed in water.
- Addition of zinc metal into a solution of copper (II) sulphate result into decolonization of the solution and deposition of a brown solid substance
(b)Define the following terms and give one example in each case
- Weak acid
- Acidic-salt
- Potassium permanganate reacted with an acid A to produce gas B.
Then gas B is passed through water and finaly to concentrated
Sulphuric acid.
- State;
- The name of compound A and gas B
- Two chemical tests of gas B
- The method which is used to collect gas B. Give reason
- Briefly explain as to why;
- Gas B is passed through water and concentrated sulphuric acid
- The preparation of gas B is always done in a fume chamber
- Write the balanced chemical equation for the reaction between Potassium permanganate and acid A
FORM FOUR CHEMISTRY EXAM SERIES 149
FORM FOUR CHEMISTRY EXAM SERIES 149
PRESIDENT OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSESSMENT
NEW EXAM FORMAT-2023
032/1 CHEMISTRY FORM FOUR
MID-TERM EXAMS MARCH – 2023
Time: 3:00 Hours
Instructions
- This paper consists of section A, B and C with a total of thirteen (13) questions.
- Answer all questions in this paper
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
- Atomic masses: H=1, C=12, O=16, N=14, Pb=108
- Avogadro’s number = 6.02 x 1023
- GMV at s.t.p = 22.4 dm3
- 1 Faraday = 96,500 coulombs
- Standard pressure = 760 mm Hg
- Standard temperature = 273 K
- 1 litre = 1dm3 = 1000cm3
SECTION A (16 marks)
Answer All Questions
1. For each of items (i) – (x), Choose the correct answer from the alternatives and write its letter beside the item number in answer sheet provided
- Mango Juice from Bakhresa was written, “Shake well before use” What does this mean?
- Suspension
- Solution
- Solute
- Emulsion
- Solvent
- Ammonia gas is collected by which method among the following
- Downward Displacement of water
- Upward delivery
- Downward delivery
- Upward Displacement of air
- The elements are required by adult plant
- What volume of hydrogen gas will be produced. When 1.3g of Zinc granules react completely with excess Dilute Sulphuric acid at S.T.P?
- 223cm3
- 130cm3
- 440cm3
- 220cm3
- 448cm3
- Which of the following substances should not be kept closely to the open bottle containing Carbon-dioxide
- Dilute Nitric acid
- Dilute Hydrochloric acid
- Sodium hydroxide solution
- Sodium Nitrate
- Dilute Suphuric acid
- When Nitrogen gas is formed covalently how many electrons are shared between nitrogen atom
- 2
- 3
- 6
- 5
- 4
- The reasons why white am hydrogen copper II sulphate tums blue when exposed in Atmosphere is that it
- Absorbs water vapour
- Reacts with Oxygen
- Reacts with carbon dioxide
- Becomes Dry
- Release water to the atmosphere
- Which action should be taken immediately after concentrated sulphuric acid is spilled on the skin
- It should be rinsed off with large quantities of running water
- It should be neutralized with concentrated NaOH
- The affected area should be wrapped tightly and shown to a medical health provider
- It should be neutralized with solid CaCO3
- It should be neutralized with concentrated KOH
- The following particles forms the nucleus of an atom
- Proton only
- Neutron and Electron and neutron
- Proton and Electron
- Proton and neutron
- Neutron and Electrons
- What should be done if results Obtained from an experiment do not support the hypothesis
- The results should be left out
- A new problem should be identified
- The experiment should be changed
- Ideas for further testing to find a solution should be given
- The hypothesis should be accepted
- Water exists in three forms, solid, liquid and vapour which among the following are examples of liquid form of water?
- Rain, Snow, hail
- Dew, Rain, Ice
- Mist, Steam, Cloud
- Rain, Hail, Ice
- Rain, Mist, Dew
2. Match the properties of element in List A with respective elements in List B by writing the letter of correct response besides item number in answer sheet provided
| LIST A | LIST B |
|
|
SECTION B (54 Marks)
Answer all questions in this section
3. (a) The modern Periodic table law is based on modification of Mendeleev Periodic law. Explain the two theories differ from each other.
(b) Comment on the following statement
- Lithium has large size than Beryllium
- Sodium is smaller than potassium
(c) Give four ions whose electron configuration resembles that of Neon
4. (a) Mr. Mwabashi asked student to prepare all requirements for extraction of sodium metal. Help them describe the use of each of the following
(i) Calcium Chloride (ii)Graphite rod (iii) Steel gauze
(b) Why is sodium collected upward in down cell?
(c) Write electrodes reaction is down cell during extraction of sodium
5. A solution contains 40.3g of substance XOH per liter 250.0cm3 of this solution required 30.0cm3 of 0.3M sulphuric acid for complete neutralization
- Calculate the number of moles of XOH that reacted
- Determine the relative atomic mass of X
6. (a) Differentiate alkanes from alkenes
(b) Study the flow chart below and answer questions that follow;
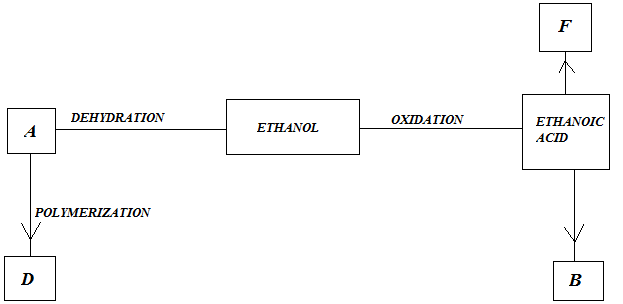 |
- Identify A
- State one Physical Property of B
- Give a reason why D pollutes the environment
- Write equation for formation of F
- Describe an experiment which can be used to distinguish buteno from butanol.
7. (a)A mass of 1.24g of a divalent metal was deposited when a current of 6A was passed through a solution of metal sulphate for 12minutes. Determine the relative atomic mass of the metal (1F = 96,500C)
(b)State two application of Electrolysis
8.(a) What is an alkali?
(b) Aqueous solution of 2M electronic acid and 2m nitric (v) acid were tested for electronic conductivity. Which solution is a better conductor of electricity? Explain
(c) Explain why it is not advisable to prepare a sample of carbon dioxide using barium carbonate and dilute Sulphuric (VI) acid
SECTION C (30 Marks)
Answer any two questions in this section
9. (a) What is Isomerism?
(b) Using illustration differentiate chain Isomerism from position Isomerism
(c) Alkenes are saturated, why alkenes are unsaturated explain
10. (a)Explain the following terms
- Reversible reaction
- Dynamic equilibrium
(b)The industrial Oxidation of sulphur dioxide is summarized as follows
2So2(g) + O2(g) → 2SO3(g) DH= -94.9 KJ/Mol
What will be the effect of each of the following on production of sulphur Trioxide?
- Increase in moles of sulphur dioxide
- Increase in pressure
- Decrease of temperature
- Decrease of moles of sulphur dioxide
(c)Briefly explain how each of the following factors affects the rate of a chemical reaction
- Temperature
- Pressure
- Concentration
- Catalyst
- Surface area
(d) Give one good reason for the following
- Fruits ripe faster during summer than during winter
- Steel wire get rust faster than iron nails
11. (a)Using Iron filling, describe an experiment that can be conducted to show that Oxygen is present in air
(b)Element U has atomic number 12 while element V gas atomic number 16. How do the melting points of elements compare
(c) In haber process, nitrogen reacts with hydrogen according to the following equation;
3H2(g) + N2(g) → 2NH3(g) DH= -92KJ/Mol
- What would be the effect of adding catalyst to the position of equilibrium
- Explain why it is not advisable to use a temperature higher than 773K in haber process
FORM FOUR CHEMISTRY EXAM SERIES 141
FORM FOUR CHEMISTRY EXAM SERIES 141
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY FORM FOUR
TIME: 2 HOURS NOVEMBER 2022
INSTRUCTIONS:
1. This paper consists of sections A, B and C
2. Answer all questions in section A and B and only one (01) question from section C
3. Cellular phones and all programmable calculators are not allowed in the examination room
4. Write your examination Number on every page of your answer booklet(s)
5. The following constants may be used
1 Faraday = 96500 coulombs
G.M.V at stp = 22.4dm 3
Avogadro's number = 6.02 x 10 23 particles per mole Atomic masses
H = 1, C = 12, O = 16, Na = 23, S = 32, cU = 64, Ag = 108, Zn = 65
SECTION A: (15 MARKS)
Answer all questions in this section
1. For each of the items (i) — (x), choose the most correct answer from among the given alternatives and write its letter in the space provided
(i) The lead in a pencil is made of a mixture of graphite and clay. When the percentage of graphite is increased, the pencil moves across the paper move easily. Which statement explain this observation?
A. Graphite has high melting point
B. Graphite is a lubricant
C. Graphite is the form of carbon
D. Graphite is a non — metal
E. Graphite is a good conductor of electricity
(ii) Which process is used to convert calcium carbonate into calcium oxide
A. Electrolysis
B. Fractional distillation ![]()
C. Cracking
D. Thermal decomposition
E. Evaporation
(iii) One of the following substance is commonly used to determine the arrangement of metals in order or reactivity
A. Water
B. Salt
C. Acid
D. Base
E. Non -metal
(iv)The hottest part of a non - luminous flame is the region of
A. Green or blue zone
B. Purple or blue zone
C. Colourless inner zone
D. Thin outer zone
E. Blue zone
(v) The following are the properties of a good fuel except
A. Average value of velocity or combustion
B. High ignition point
C. Highest energy value
D. Environmental friends
E. Less or no waste products
(vi) A sodium atom (Na) and a sodium iron (Na+) have the same
A. Atomic site
B. Electronic configuration
C. Physical properties
D. Physical properties
E. Number of protons
(vii)Factors in an experiment that remain unchanged throughout the experiment is known as
A. Controlled variable
B. Dependent variable
C. Independent variable
D. Uncontrolled variable
E. Fixed factor
(viii) Coal, methane and hydrogen are burned as fuel, which description of this process is correct?
| What happens to the fuel | Type of reaction |
| Endothermic Exothermic Endothermic Exothermic Displacement |
(ix)Petroleum is a mixture of different hydrocarbons, which process is used to separate petroleum into groups of similar hydrocarbons?
A. Combustion
B. Cracking
C. Fractional distillation
D. Reduction
E. Isomerism
(x) A car releases 5g of carbondioxide gas into air for each mile driven. The number of carbondioxide molecules released are
A. 1.0 x 1023
B. 6.8 x 1023
C. 6.3 x 1023
D. 5.8 x 1022
E. 6.8 x 1022
2. Match the item in LIST A with a correct response in LIST B by writing the letter of tie correct response below the corresponding item in the table G![]() iven
iven
| LIST A | LIST B |
|
|
SECTION B: (70 MARKS)
Answer all questions in this section
3. (a) Element Q has 17 electron and 18 neutrons (i) What is the atomic number of element Q
(ii) What is the mass number of element Q
(iii) Write down the electronic configuration of element Q
(iv) In which group and period in the periodic table does element Q occupy?
(b)Given the following 204J 208 K 207L and AM are isotopes of element H, whose abundances are 2%, 24%, 22% and X% respectively. Calculate the abundance X% and the mass number A of isotope M, given that the atomic mass of element H is 207.
4. (a) State any three main physical properties of water and show the usefulness of each property.
(b)The following methods were used to soften 25cm 3 of the original sample of water. At the end of each experiment, the resultant product was treated with soap solution until lather was obtained
| Method used | Volume required for 25cm3 0f product |
| Filtering | 20 |
| Distilling. | 1 |
| Adding Na2CO3 washing soda | 1 |
| Boiling | 20 |
| Adding zeolite | 1 |
(i) Which methods were most suitable for softening hard water?
(ii) Which type of hardness was present in the sample provided? Explain
(iii) Which substances caused the hardness in the sample water?
(iv) Why did distillation and filtration produce such different results?
5. (a) Outline three reasons to explain why carbondioxide is used to extinguish fire
(b)A sample of mass 28.6g of hydrated sodium carbonate (Na2 C03. 101420) was heated such that water was entirely absorbed by 32g of anhydrous copper (Il) sulphate to form a blue compound of hydrated copper (Il) sulphate (CUS04 . XH20). Find the value of x in the copper (Il) sulphate
6. (a) Complete and balance the following chemical equation.
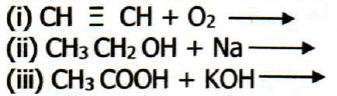
(b) Write down the condensed structural isomers of alcohol of molecular formula C4H100
7. (a) By using two examples in each case, categorize fuel according to their physical state
(b) What is the simplest chemical formula of a compound formed when 36g of magnesium combine with 14g of nitrogen?
8. (a) Complete the following table
| Element | When burned forms | Kind of oxide | Action of oxidation water | Action on litmus |
| Carbon | Co2 |
|
|
|
| Sodium |
|
| NaoH |
|
| Sulphur |
| Acidic |
| Blue to red |
(b) Using calcium oxide and carbon dioxide explain the meaning of
(i) Acidic oxide (ii) Basic oxide
9. A piece of marble chips (calcium carbonate) was placed in a beaker containing an excess of hydrochloric acid standing on a reading balance. The mass of the beaker and its contents were recorded after eve 2 minutes as shown in the diagram below
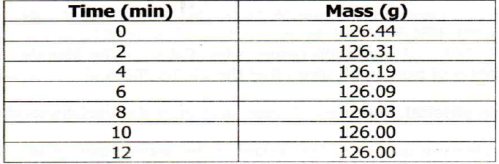
(a) Why was there a loss of mass?
(b) Write the equation for the reaction above
(c) State three (3) different ways in which reaction could have been more rapid
![]() (d) Why did the mass remain constant after 10 minutes?
(d) Why did the mass remain constant after 10 minutes?
10. (a) Nowadays the use of spatula which are made of iron is discouraged in the laboratory. Explain one reason for the scenario.
(b) Write a chemical composition for the following fire extinguisher
(i) Dry powder (ii) Wet chemical
(c) How can you prevent the following iron materials from rusting?
(i) Gates
(ii) Bicycle chain
(iii) Iron sheets
11. (a) How can a mixture of two or more miscible liquids be separated from one another?
(b) Make a flow chart to show the separation of a mixture of common salt and sand
12. Four experiment were conducted using chemicals P, Q, L and M on different materials.
The results were given as indicated below. You are required to write the correct terminology which suits each of the results below and give precautions of handling the warning signs which goes with it.![]()
(i) When chemical P was poured on the wood it completely damage the surface of the wood
(ii) When chemical Q was poured on the small fire, it found burst and turning a bigger fire
(iii)When chemical L was swallowed by a rat, the rat died immediately
(iv)When chemical M was exposed on air sudden blast accompanied with light occurred (b) Give one main importance of chemical warning signs
SECTION C: (15 MARKS)
13. (a) State Faraday's first law of electrolysis
(b)The apparatus shown below was used in the experiment of electroplating a key with silver. The electrolyte used is silver nitrate and an electric current of 15A was passed in the electrolyte for two and half hours. Study it carefully and answer the questions that follows;
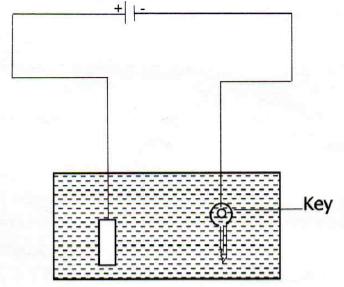
(i) Name the process taking place on key as either oxidation or reduction
(ii) With the current of 15A, what mass of silver was deposited on the key?
(iii) Name the gas formed at the anode![]()
(iv) Name the acid formed as a result of electrolyzing silver nitrate
(v) What volume of the named gas in (iii) above was collected in this experiment?
14. (a) Eutrophication is excessive growth of aquatic plant and algae in water bodies (i) Give two causes of eutrophication
(ii) Explain the effect of eutrophication. (Give two points)
(b)Explain how industrial workers can be protected against harmful effect of the chemicals. (Give three points)
FORM FOUR CHEMISTRY EXAM SERIES 129
FORM FOUR CHEMISTRY EXAM SERIES 129
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY
FORM FOUR- SEPT 2022
CODE 032/1
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of fourteen (14) questions
- Answer all questions in sections A and B and one (1) question from section C
- Sections A and C carry fifteen (15 ) marks each and section B carries seventy (70) marks
- Cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Atomic masses H=1, C=12 , N=14, O=16 , Cl=35.5 , CU=64 , P=15, Fe=56
Avogadro’s number =6.02 x 1023.
GMV at s.t.p= 22.4 dm3.
1 Faraday = 96,500 coulombs.
Standard pressure =760 mm Hg.
Standard temperature =273 K.
1 litre = 1 dm3 =1000 cm3.
SECTION A (15 Marks)
Answer all questions in this section
- For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- A metal nitrate which will not give a metal oxide on heating is:
- Calcium nitrate
- Silver nitrate
- Lead nitrate
- Copper nitrate
- Zinc nitrate
- What number of faradays of electricity is required to deposit 4g of calcium from molten calcium chloride?
- 0.1
- 0.2
- 0.4
- 0.3
- 0.7
- When methane undergoes substitution reaction with excess chlorine. What is the final product?
- Chloromethane
- Dichloromethane
- Trichloromethane
- Tetracloromethane
- Monochloromethane
- The reason why white anhydrous copper (II) Sulphate turns blue when exposed in Atmosphere is that it
- Absorbs water vapour
- Reacts with oxygen
- Reacts with carbon dioxide
- Become dry
- Release water to the Atmosphere
- Which action should be taken immediately after concentrated sulphuric acid is spilled on the skin?
- It should be rinsed off with large quantities of running water.
- It should be neutralized with concentrated NaOH
- The affected area should be wrapped tightly and shown to a medical health provider
- It should be Neutralize with solid CaCO3
- It should be neutralized with concentrated KOH
- The molarity of 5.3g in 100ml of Na2CO3 solution is
- 0.2M
- 0.5M
- 0.05M
- 0.005M
- 0.01M
- The reaction between hydrogen and Iodine is represented by H2(g) + I2(g)
 2HI(g)
2HI(g)  H=-xKJ/mol The reaction is
H=-xKJ/mol The reaction is
- Endothermic reaction
- Neutralization reaction
- Displacement reaction
- Exothermic reaction
- Decomposition reaction
- One of the following apparatus is used to measure fixed volume of liquid
- Burette
- Pipette
- Conical flask
- Measuring cylinder
- Volumetric flask
- A substance which absorb water from the atmosphere and form a solution is called
- Efflorescent
- hygroscopic
- deliquescent
- amphoteric
- salt
- AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNo3(aq). This a good example of
- Neutralization reaction
- double decomposion reaction
- Redox reaction
- synthesis reaction
- decomposition.
2. Match the properties of element in list A with the respective element in List B by writing the letter of the correct response besides the item number in answer sheet provided.
| LIST A | LIST B |
| (i) A black solid element which burns with reddish glow giving off colorless gas which is slightly acidic (ii) Silvery white metal which burns with golden yellow flame giving an oxide which is basic in nature (iii)A yellow element in color which burns with blue flame giving colorless gas which is strong acidic in nature (iv) A shinning white strip metal burns with dazzling white flame giving an oxide which is slightly basic in nature (v) A silvery white metal which burns with brick red giving off oxide which is white solid
|
|
3. (a) The Modem periodic law is based on modification of Mendeleev periodic law. Explain how the two theories differ from each other.
(b) Comment on the following statement
(i) Lithium has large size than Beryllium
(ii) Sodium is smaller than potassium
(c) Give any four ions whose electronic configuration resemble to that of Neon. (07 Marks)
4. a) How many chlorine molecules are in 20cm3 of chlorine gas at s.t.p.
b) Calculate number of ions present in 5g of copper (II) Nitrate
5. (a) (i) People suffering from heart burn usually use wood ashes for relief. Mention chacteritics which makes the ashes to be used for heart burn relief.
(ii) Give four compounds found in the laboratory which show the same characteristic as ashes.
(b) How many ions are there in 6.82g of Al2(SO4)3
6. 5.3g of X2CO3 was dissolved in water to make 0.5 litre of a solution. 25cm³ 0f this solution required 50.0cm³ of 0.1M HCl for complete neutralization.
a) Write the balanced chemical equation for the reaction
b) Calculate the concentration of X2CO3 in mol/dm3
c) Calculate the relative molecular mass of X2CO3
d) Calculate the relative atomic mass of X
e) What is the name and symbol of element X
7. (a) A gaseous compound consists of 86% Carbon and 14% Hydrogen by mass. At S.T.P, 3.2dm3 of the compound had mass of 6g.
(i) Calculate its molecular formula
(ii)Give the IUPAC name of the compound
(b) Most of the apparatus in the laboratory are made up of glass materials. Support this statement by giving ant two (2) reasons. (07 Marks)
8. Element R having atomic number 20 combines with element S having atomic number 17 to form a certain compound
a) Write the formula of the compound and state the type of bond formed in the compound
b) Give any three properties of the compound formed in 7(a) above
9. a) Muumini is a student from Katosani secondary school, she is not able to State Le Chatellier’s principle, Help her.
b) In the industrial preparation of Sulphur trioxide mr Atieno yo established equilibrium between Sulphur dioxide and oxygen gas as follows:
2SO2(g) + O2(g) ![]() 2SO3(g)?H= - 94.9KJ/mol.
2SO3(g)?H= - 94.9KJ/mol.
i. How would you adjust temperature and pressure to maximize the proportion of the product at equilibrium?
ii. Why is it unfavorable to work with very high pressure and very low temperature in the contact process?
iii. What catalyst is used to speed up the rate of formation of Sulphur trioxide before attaining the equilibrium?
10. a) Mr Mapulishi tasked to prepare all requirements for extraction of sodium.Describe the use of each of the following during extraction of Sodium
i. Calcium Chloride ii. Graphite anode
iii. Steel gauze
b) .Why sodium is collected by upwards in the downs cell
c) Write electrodes reaction in downs cell during extraction of Sodium
11. Mr Kalubandika wanted to know some chemistry pertaining concepts. Help Mr Kalubandika to answer the following conceptual questions.
a) In which other areas do we find the warning signs out of laboratory (give four point)
b) Explain how measurements of volume differ when using measuring cylinder and burette
c) It is recommend that laboratory apparatus should be properly washed or wiped after use, explain the significance for this when
i. Measuring volume of liquids
ii. Measuring mass of substance
12. (a) Give the meaning of the following terms
(i) Soil pH (ii) Liming
(b) (i) Explain why sulphur and its compounds are removed from the fuel before they are burned
(ii) By using a reaction equation explain how propane differs from propene
13. (a) Outline any three uses of carbon dioxide gas in animals and plants
(b) Complete the following reactions
(i) CH2=CH2 + O2![]()
(ii) CaC2 + 2H2O ![]()
(iii)CH4 +Cl2 ![]()
(iv) C2H5OH +O2 ![]()
14. In q certain chemical plant that deals with the production of hydrogen gas, a certain chemist passes an electric current in series through copper (II) Sulphate and sulphuric acid solutions. In both electrolytes, inert electrodes were used. If 8400cm3 of hydrogen at S.T.P were produced for several hours, what volume of oxygen gas would also be produced? What mass of copper was produced?
FORM FOUR CHEMISTRY EXAM SERIES 119
FORM FOUR CHEMISTRY EXAM SERIES 119
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY -SEPT 2022
FORM FOUR
CODE 032/1
CHEMISTRY 1
TIME:3HOURS SEPT 2022
INSTRUCTIONS
- This paper consists section A, B and C with a total of fourteen questions.
- Cellular phones and any unauthorized materials are allowed in the examination room.
- Non – programable calculator may be used in the examination room.
- Write your examination number on every page of your answer booklet(S).
- The following constant may be used.
Atomic masses H = 1, O = 16, S = 32, Zn = 65, Fe = 56, Cu = 64, Ca = 40.
Avogadro’s number = 6.02 1023
GMV at s.t.p = 22.4dm3
1 Faraday = 96500 coulombs
1Litre = 1dm3 = 1000cm3
SECTION A (15 Marks)
Answer all questions in this section
- For each of the items (i) – (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- The element with a proton number 17, has similar chemical properties to the element with the proton number _______
- 7 (b) 9 (c) 16 (d) 19 (e) 17.5
- Which statement about catalyst is correct?
- Are used in industry to reduce energy cost
- Are used up during a reaction
- Increase the activation energy of chemical reactions
- Transition metals are not good as catalyst
- Used to form products of reaction
- A man suffering from excess of acid in the stomach has no indigestion tablets. Which substance could he take to lower his acidity?
- Asprin, PH 6 (b)Bicarbonate of soda, PH8
( c)Lemon juice, PH 5 (d)Salt water, PH 7
( e)Orange juice PH 4
- Excess zinc carbonate reacts with dilute hydrochloric acid according to the equation shown below.
ZnCO3(s) + 2HCl(aq) → ZnCl2(aq) + CO2(g) + H2O(l)
What volume of carbon dioxide gas is produced at S.T.Pto form 0.1 mole of the acid?
- 1.2dm3 (b) 2.4dm3 (c) 12dm3 (d) 24dm3 (e) 4.5fm3
- A pupil dissolved some ammonium nitrate crystals in water as shown below.
Before After
What type of reaction took place above?
- Endothermic reaction (b)Exothermic reaction
( c)Neutralization (d)Reduction reaction (e)Resolution reaction
- Which of the following is not a commercial use of hydrogen gas?
- Manufacture of ammonia (b)Used to support lives of living things
(c)Manufacture of margarine (d)Formation of water (e)As rocket fuel
- A student read the following statements on an article about how carbon dioxide is formed.
- From the fermentation of glucose
- When calcium carbonate reacts with dilute hydrochloric acid
- When methane burns in a limited supply of oxygen
Which of these statements are correct?
- 1, 2 and 3 (b) 2 and 3 only (c) 1 and 3 only
(d)1 and 2 only (e) 3 and 2 only
- An acid differs from a base in that an acid.
- Turns a red litmus paper (b)Has a PH value above 7
(c)Has a sour taste (d)Turns a blue litmus paper red
(e)Does not react with base
- Methane is a green house gas. Which process releases methane into the air?
- Combustion
- Decay of vegetable matter
- Volcanic activity
- Photosynthesis
- Decantation
- The following role is played by organic matter in the soil.
- Improving water infiltration of the soil
- Accelerating break down of organic matter
- Reserving nutrients thus providing soil fertility
- Converting nitrogen into nitrate
- Providing a room for organic materials such as nylon
- Match the description in list A with the corresponding response in list B by writing the letter of the correct response besides the item number in the answer booklet/sheet.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions from this section
- (a) As a chemist what factors would you consider in selecting a good fuel? (5points)
- Why it is not advisable to sleep inside a house which is not well ventilated with a burning wooden charcoal? Support your answer with chemical equation. (7 marks)
- (a) (i)What is oxidation number of iron in iron (III) chloride?
(ii) In the following reaction state the substance which is reducing agenda and oxidizing agent.
H2 (g) +CuO(S) → Cu (g) +H2O (l)
(b) Classify the following reaction into oxidation and reduction reaction.
Fe3+(aq)+ e → Fe2+(aq)
(ii) Fe2+(aq)+ e → Fe3+(aq)
(iii) S(s) + O2(g) → SO2(g)
(iv) N2(g) + 3H2(g) → 2NH3(g) (7 marks)
- A spillage of 15.5kg of sulphuric acid results from an accident of a road tanker. Slaked lime is used to neutralize the acid according to the equation below.
H2SO4 (aq) + Ca(OH)2(aq) → CaSO4(aq) + H2O(l)
- Balance the equation above
- Determine the molar mass of Ca(OH)2
- Use the balanced equation to determine the mass of calcium sulphate formed during the neutrazation of the spilt acid.
- Calcium hydroxide is base, which ion present in the compound is responsible for its basic properties?(7marks).
- (a) Most metals are not found as pure elements in the earth’s crust, and iron is one such metal. Iron is exctracted from its ore in a blast fumace.
- Name two other raw materals added to the blast fumace other than haematite.
- Write abalanced chemical equation for the reduction of the iron ore to the metal.
(b) State three conditions necessary for rusting to occur.(7marks)
- (a) Use the following list of elements to answer the equations below iron, lethium, mercury, oxygen, potassium, sulphur.
- Is used as catalyst in the manufacture of ammonia in the habor process.
- Is lower than sodium in the reactivty series.
- Is a non – metallic solid, whose atoms contain only six valency electrons.
- Is in period 4 of the periodic table.
- Forms an oxide which is amphoteric.
(b) Explain how the knowledge of chemistry is applied in the following field.
(i) Pharmacy (ii) agriculture (7marks)
- (a) Why it is important to treat and purify water? Five points.
(b) Give out the role of the following chemicals in water treatment and purification.
- Chlorine
- Aluminiun sulphate(7 marks)
- (a) The choice of indicator during titration depends on the properties of the reactants. State the suitable indicator in the following titration.
- Ethanoic acid + ammonium solution
- Sodium hydroxide + ethanoic acid
- Potassium hydroxide + hydrochloric acid
- Hydrochloric acid + ammonia solution
(b) In volumetric analysis not all reactions are suitable for titration. Give out three conditions necessary for titration. (7 marks)
- (a) The amount of subsatances liberated at electrodes during electrolysis depends on different factors. State three factors according to the statement above.
(b) 0.4 faradays of electricity were passed through solutions of copper (II) sulpahete and dilute sulphuric acid. Calculate the volume of hydrogen gas produced at S.T.P.(7amrks)
- (a) Give an account for the following.
- Anhydrous copper (II) sulphate becomes coloured when exposed to the air for a long time.
- Carbon dioxide gas can be collacted by down ward delvery method.
- Concentrated sulphuric acid is not used for drying hydrogen sulphide gas.
- Sodium metal is kept in paraffin oil.
(b) Write all possible isomers of (C5H8).(7marks)
- (a) Study the following equation carefully and answer the questions below.
- Explain three factors which would maximize the yield of ammonia.
- How equilibrium state can be attained.
(b) Draw the warning sign that should be put to the container caring the following chemicals.
(i) Concentrated sulphuric acid.
(ii) Zinc sulphate crystals (7marks)
SECTION C (15Marks)
Answer only one question from this section
- Many countries this year have experienced shortage of rainfall that has resulted to poor yield of crops and death of animal due to lack of water and pastures. Scientists have advised that the problem is due to air pollution in many countries in the world. Explain what might happen in the future to the world if the present trend of polluting the air is not controlled and checked. (Six points)
- With the aid of diagram describe how sulphur is extracted from its deposit.
1 | Page
FORM FOUR CHEMISTRY EXAM SERIES 113
FORM FOUR CHEMISTRY EXAM SERIES 113
THE PRESIDENT�S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM FOUR-2022
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14�questions
- Answer all questions in section A and B�and ONE (1) question from section C.
- Section A and C carries 15 marks, while�section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro�s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3�= 1000cm3
�SECTION A ( 15 Marks)
Answer all questions in this section
1. For each of the items (i)-(x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- The percentage by mass of sodium in Na2 CO310H2O is:-
- 16.08%
- 18%
- 23%
- 40%
- 80%
- Which of the following lists contains renewable sources of energy?
- Biogas, solar power and charcoal
- Hydro electric power, coal and battery
- Biogas, solar power and firewood
- Charcoal, petrol and tidal waves
- Biogas, solar power and tidal waves
- Equal volumes of water form five different sources are boiled in a dish until no water is left. Which of the following will leave behind no deposits?.......................
- Spring water
- Rain water
- Lake water
- Pond water
- Tap water
- A saturated blue solution was electrolyzed using carbon electrodes. A reddish brown layer was formed on cathode and a reddish brown liquid at anode which of the following solutions was electrolyzed?
- Iron (III) chloride
- Copper (II) sulphate
- Iron (II) bromide
- Copper (II) bromide
- Iron (III) sulphate
- The chemical formula of carbon dioxide is CO2. The number 2 beside O means that there___________
- Are two molecules of carbon dioxide
- Is half an atom of oxygen for each molecule of carbon dioxide
- Are two atoms of oxygen for each molecule of carbon dioxide
- Are two atoms of carbon for each molecule of carbon dioxide
- Are two molecules of oxygen
- Below is a list of general formulae of homologous series of some organic compounds 1. C2H2n+1OH��� � � � �2. CnH2n+2� �3. CnH2n+1�COOH �4. CnH2n-2� .The correct order of the formulae is,
- Alkanoic acids, alkanes, alcohols and alkenes
- Alcohols, alkenes, alkanoic acids and alkanes
- Alcohols, alkanes, alkanoic acids and alkenes
- Alcohols, alkynes, alkanoik acids and alkenes
- Alkanes, alcohols, alkanoic acids and alkenes
�
- Your friend�s clothes catch fire. Which of the following is most effective in extinguishing the fire?
- Wet sand
- Wet blanket
- Carbon dioxide extinguisher
- Foam extinguisher
- None of the above
- Which of the following pair of particles has the same electronic structure?
- Sodium atom and chloride ion
- Carbon atom and chloride ion
- Calcium atom and chloride ion
- Chloride ion and neon atom
- Chloride atom and neon ion
- Exactly 50cm3 of a gas weighs 0.125g at S.T.P the relative molecular mass of the gas must be :-
- 56
- 44
- 22.4
- 100
- 32
- Which of the following fertilizers can be applied to neutralize the pH of slightly alkaline soil?
- Ammonium sulphate
- Urea
- Farmyard manure
- Compost manure �E.sisal wastes.
- Match the items in list A with the correct responses from list B
| List a� | List b |
|
|
�
�
�
�
�
�
SECTION B (70 marks)
Answer all questions in this section
- (a) (i) What observable changes occurs when hydrated iron (II) sulphate crystals are heated mildly and then strongly in a test tube?
(ii) Write two balanced equations for the above reactions
(b) When crystals of hydrated sodium carbonate (Na2CO3xH2O) where heated strongly only 37.06% by weight of the salt remained. How can you show that the value of x in Na2CO3 .xH2O is 10?
- I. �A Green solid T was heated until there was no further change. The following observations were made;
- Colourless liquid condensed on cooler parts of the test-tube
- A colourless gas which turns acidified potassium dichromate(VI) green was formed
- Red-brown residue S was formed
- Identify solid T
- Name the substance that could be used to identify colorless liquid
- Name the residue S
- Write equations for reactions that took place
- Why is iron not used to make steam boilers?
Study the flow chart below and answer questions that follows.
- What are the identities of solution A and solid C? Write chemical name and formulae
- What the day to day use of the conversion marked 2?
- (a) What is the role of a catalyst in a chemical reaction?
(b) Draw an energy level diagram for the decomposition of potassium chlorate when a catalyst: (i) is used (ii) not used
6. � (a) � �Briefly explain the concept of scientific procedure.
(b) � What is the importance of the scientific procedure in daily life? Give two points.(7 marks)
7.��Study the information in the table below and answer the questions that follow;
| Element� | Atomic number | Boiling point |
| A | 3 | 1603 |
| B | 13 | 2743 |
| C | 16 | 718 |
| D | 18 | 87 |
| E | 19 | 1047 |
�
- Select the elements which belongs to the same period and group
- Which element is
- Gaseous at room temperature
- Does not form an oxide, give a reason
- Write the formula of nitrate of B
�
8(a) Why is a fertile soil not necessarily productive?-give 2 points.
(b)(i) why is liming material added to soil?
� � �(ii) What are the causes that make the soil in need of liming material?-give 2 points.
9(a) Why do iron window frames rust quickly even though they are painted while aluminium� � �frames are resistant to rusting?
(b)Why are aluminium vessels used to transport nitric but not sulphuric acid?
10(a) (i) What is meant by fuel?
� � �(ii) How many classes of fuels are there?
(b)(i) The traditional process of making charcoal in an earth mould or pit kiln is wasteful process. Briefly explain.
� � ��ii)what is the effect of using charcoal on the environment?
11(a) (i) What is the relationship between athanoic acid and ethanol?
� � �(ii) How does the acidity of ethanoic differ to that of sulphuric acid?
(b)How does ethanoic acid react with:- (i)sodium metal? (ii) sodium carbonate solution?
SECTION C (15 MARKS)
Answer all question in this section.
12. During electrolysis one ion is discharged at each electrode. When two or more ion of similar charge are present in an electrolyte, only one ion is selected for discharge.
The selection depends on different factors. Explain how these factors affect the selection. Giving examples and relevant equations in each case.
13.You are in Amboni district in Tanga region where there is potential sulphur deposit. The district development society aims to extract the sulphur and has come to you for advice. Explain to the society.
- The equipment needed to the extraction. Use an illustration to make the explanation clear.
- The process involved during and other the extraction
- The effect of extraction on the environment
- Commercial uses of sulphur � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � ��
13.���(a)����Give three ways in which environmental destruction is likely to occur during extraction of metals.
(b)���The following equations represent the steps involved in the conversion stages of iron extraction in Bussener converter. Arrange the equations in chronological order from the first step to the last by writing the respective letter so as to get a complete explanation of the conversion stage.
�V: 2Cu20(S) +���Cu2S(S)?�����6Cu (l) + S02�(g)
�W:��FeO (l) + SiO2(g) ? FeSiO3
�X: 2Cus(s) +302�(g)�?����������2Cu20(s) +2S02�(g)
�Y: 2FeS (l) +302�(g) ? 2Feo(1) + 2S02�(g)���
�
1
�
14. Using a well labelled diagram, explain how sulphur is extracted using the Frasch process
FORM FOUR CHEMISTRY EXAM SERIES 88
FORM FOUR CHEMISTRY EXAM SERIES 88
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM FOUR-MARCH/APRIL-2022
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (15 Marks)
Answer All questions in this section.
- The method of collecting hydrogen chloride gas in a class experiment is known as:
- Downward displacement of water
- Downward displacement of air
- Upward displacement of air
- Fountain
- Condensation
- The only metal which does not react with dilute hydrochloric acid is:-
- Magnesium
- Copper
- Zinc
- Sodium
- calcium
- Which among the following equations correctly shows the reaction between chlorine gas and water?
- C l2(g) + H20(1)→CI2(g)
B 2C12(g) + 2H20(1)→ 4C1-1(aq) + 02(g) + 2H2(s)
- Cl2(g) + H20(1)→HCl + HOCI(aq)
- 2Cl2(g) + 2H20(I) →2H0C1 +H2(g)
- 2C12(g)+ 3H200) → C12 (.0 + 2H30+
- A gas which when exposed to air forms white fumes is likely to be:
- Nitrogen
- Chlorine
- Ammonia
- Hydrogen chloride
- Sulphur.
v. Which is not true about hydrogen chloride?
- It supports combustion
- It is a very soluble gas
- It forms white fumes with ammonia
- It is acidic in nature
- When exposed to air forms white fumes
- Sea water contains various salts. Which salt is present in the largest proportion?
A. Magnesium sulphate
B. Sodium chloride
C. Calcium sulphate
D. Magnesium chloride
- Study the chemical equations below: which can remove temporary and permanent hardness?
a. Ca(HCO3)2(aq) → CaCO3(g) + H2O(l) + CO2(g)
b. Ca(HO)2(aq) + Ca(HCO3)2(aq) → CaCO3(g) + 2H2O(l)
c. Ca2+(aq) + CO2-(aq) → CaCO3(g)
d. Ca2+(aq) + Na2Z(aq) → 2Na+(aq) + CaZ(g)
- Hard water which is softened just by boiling contains dissolved;
A. Calcium carbonate
B. Calcium chloride
C. Sodium carbonate
D. Magnesium sulphate
E. Calcium hydrogen carbonate
- Which of the above equations (vii) removes temporary hardness of water only?
A. a and b
B. b and c
C. c and d
D. d and a.
- Which set of compounds when in water cause hardness easily removed by boiling addition of Na2CO3 or use of ion exchange?
A. Ca(HCO3)2, Mg(HCO3)2
B. CaCl2, MgSO4
C. Fe(NO3), Ca(NO3)2
D. mgCl2, FeCl2
2. Match the physical processes represented by arrows (i) - (v) in List A with the corresponding terms in List B by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
| i) Water that easily forms lather with soap ii) Water that does not form lather with soap iii) Water that contains dissolved calcium and magnesium hydrogen carbonate iv) Water that contains dissolved sulphates of calcium and magnesium v) An element whose complex ion is used in softening water
| A. Plaster of Paris B. Gypsum C. Nitrogen dioxide D. Carbon dioxide E. Calcium F. Phosphorous G. Soft water H. Hard water I. Permanent hard water J. Temporary hard water K. Scum L. Stain M. Fur N. Coating O. Sodium P. Potassium Q. magnesium |
3. Figure 1 below represents the laboratory preparation of hydrogen chloride gas.
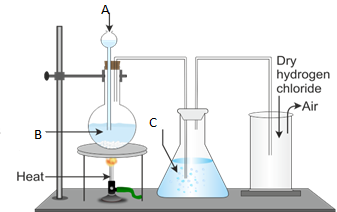
(a) Name the parts labelled A, B, C and D.
(b) (i) Do you think the gas can be collected over water? Give reasons for your answer.
- Explain the test for the gas.
- What is the function of C?
- Name the method used to collect the gas.
- Write a balanced chemical equation for the reaction taking place during the preparation of hydrogen chloride gas.
(c) Write chemical equations for the reaction between:
(i) Ammonia gas and hydrogen chloride.
(ii) Hydrogen chloride gas and water.
4. Write equation for reaction between
- Chlorine and Magnesium
- Chlorine and phosphorous
- Chlorine and copper
- Chlorine and hydrogen sulphate
5. Explain what happens when a stream of Hydrogen chloride gas is passed over
- Ammonia gas
- Iron II solution.
6. Study the diagram below and answer the questions that follow
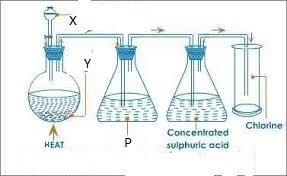
The apparatus above are used to prepare chlorine
- State substance
Y
X
P
- What is the use of conc. Sulphuric acid?
- State the use of p.
- Write equation for reaction occurring at the flask.
- How can you show that test tube used for collection of chlorine is full?
- Name the method of collection
- Give two uses of chlorine
- State 2 compounds of chlorine that pollute the environment.
7. Write down the chemical equations used when softening water of the
(a) Temporary hardness through (i) boiling water (one question) (ii) use of chemicals (two equations)
(b) Permanent hardness through (i) use of chemicals (one equation) (ii) iron exchange (one equation)
8. Define the following terms;
(e) Soft water
(f) Hard water
(g) Permanent hardness of water (h) Temporary hardness of water
9. a) What is the hardness of water?
b) Briefly explain types of hard of water.
c) State the causes of hardness of water for each type mention in (b) above.
d) Explain how you would remove the hardness of water according to its type.
e) Give three (3) advantages and three (3) disadvantages of the hard water.
10. Balance the following equations:
(i)Ca + H3PO4→ Ca3(PO4)2 + H2
- Cu + HNO3 → Cu (NO3 )2 + NO2 +H20
- SnCi2+FeC13→SnC14+FeCI
11. Give the name of the types of reaction represented by each of the following chemical equations.
- C3H8(g) +50,(0)→ 3CO2 + 4H20(1)
- 2Pb (N 03),(,)→2Pb0(,) + 4NO2 +02(g)
(iii)Zn(s)+CuS04(aq) —>ZnSO4(aq) +CU(S)
12. Complete the following equations and determine the type of chemical reaction involved in each case.
(i) Zn(s)+ H2SO4(aq)→
(i) AgN 03(aq) + NaCl(aq)→
(iii) N2(g) + H2(g) →
FORM FOUR CHEMISTRY EXAM SERIES 74
FORM FOUR CHEMISTRY EXAM SERIES 74
THE UNITED REPUBLIC OF TANZANIA
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM FOUR MID TERM EXAMINATION-2021
CHEMISTRY
Time 3:00 HrsAUG, 2021
| Instructions
Atomic masses: H=1, O=16, S=32, Ca=40, Zn=65, Cl=35.5, Na=23, C=12, Ag=108 Avogadro’s number = 6.02 x 1023 GMV at s.t.p = 22.4dm3 1 faraday = 96,500 coulombs 1 litre = 1dm3 = 1000cm3 |
SECTION A (15Marks)
Answer all questions in this section
- For each of the items (i) – (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- What number of faradays of electricity is required to deposit 4g of calcium from molten calcium chloride?
- 0.1 B. 0.2 C. 0.4 D. 0.3 E. 0.7
- When methane undergoes substitution reaction with excess chlorine. What is the final product?
- Chloromethane
- Dichloromethane
- Trichloromethane
- Tetracloromethane
- Monochloromethane
- Which name is given to the uniform mixture of metals?
- Compounds B. Alloys C. Ores D. Salts E. Mixtures
- The reaction between iodine and hydrogen is represented by the following equations
![]() I 2(g) + H 2(g) 2HI (g)
I 2(g) + H 2(g) 2HI (g)![]()
The reaction is:-
- An endothermic reaction
- A displacement reaction
- A neutralization reaction
- A thermal decomposition reaction
- An exothermic reaction
- The electronic configuration of this element Cl- is
- 2:8:7 B. 2:8:8 C. 2:8:8:1 D. 2:8:8:2 E. 2:8:6
- Glucose dissolves in water to form a uniform mixture.
- water is solution, glucose is solvent and the product is solute
- water is solute, glucose is solvent and the product is solution
- water is solvent, glucose is solute and the product is solution
- water and glucose forms immiscible mixture
- water and glucose form emulsion
- The reason why white anhydrous copper (II) Sulphate turns blue when exposed in
Atmosphere is that it
- Absorbs water vapour
- Reacts with oxygen
- Reacts with carbon dioxide
- Become dry
- Release water to the Atmosphere
- In order to produce the greatest amount of hydrogen in a short time, one gram of magnesiumribbon should react with
- 10cm3 of 0.5M Sulphuric acid
- 40cm3 of 0.5M acetic acid solution
- 40cm3 of 0.5 Sulphuric acid solution
- 20cm3 of 1M Sulphuric acid solution
- 20cm3 of 1M acetic acid solution
- Which of the following substances represent a group of acid oxides?
- Carbon dioxide, carbon monoxide and Sulphur dioxide.
- Sulphur trioxide, Nitrogen dioxide and Nitrogen monoxide
- Carbon dioxide, Sulphur dioxide and dinitrogen oxide
- Sulphur trioxide, carbon dioxide and Nitrogen dioxide
- Carbon monoxide, nitrogen oxide and sulphur dioxide.
- Which action should be taken immediately after concentrated sulphuric acid is spilled onthe skin?
- It should be rinsed off with large quantities of running water.
- It should be neutralized with concentrated NaOH
- The affected area should be wrapped tightly and shown to a medical health provider
- It should be Neutralize with solid CaCO3
- It should be neutralized with concentrated KOH
- Match the items in List A with the response in List B by writing the letter of the corresponding responses beside the item number.
| LIST A | LIST B |
|
|
SECTION B (70Marks)
Answer all questions in this section
- (a)
- What is laboratory?
- Name two main compound used in preparation oxygen gas in laboratory
- Why hydrogen gas is collected by downward displacement method?
(b) Complete and balance the following chemical reaction
- NaOH(ag) + HCl(ag)→
- NH4Cl(ag) +NaOH(ag)→
- Ca(OH)2(ag) +H2SO4(ag)→
- Mg(s) +HNO3(ag)→
- Element R having atomic number 20 combines with element S having atomic number 17 to form a certain compound
- Write the formula of the compound and state the type of bond formed in the compound
- Give any three properties of the compound formed in 7(a) above
- a) How many chlorine molecules are in 20cm3 of chlorine gas at s.t.p.
b) Calculate number of ions present in 5g of copper (II) Nitrate
- a) State LeChatellier’s principle.
b) In the industrial preparation of Sulphur trioxide equilibrium is established between Sulphurdioxide and oxygen gas as follows:
2SO2(g) + O2(g)?2SO3(g)?H= - 94.9KJ/mol.
- How would you adjust temperature and pressure to maximize the proportion of the productat equilibrium?
- Why is it unfavorable to work with very high pressure and very low temperature in thecontact process?
- What catalyst is used to speed up the rate of formation of Sulphur trioxide beforeattaining the equilibrium?
- a) What is molecular formula?
- You are provided with a compound composed of 22.2% Zinc, 11.6% Sulphur, 22.3% oxygen and the rest percentage is water of crystallization. Calculate the molecular formula of the compound if its molecular mass is 283.
- In one titration experiment 25cm3 of hydrated sodium carbonate Na2CO3.XH2O were titrated against 20cm3 of 0.25M hydrochloric acid. The solution of sodium carbonate was made by dissolving 7.15g of the compound in 250cm3 of distilled water.
Use the above data to answer the following questions
- Write down a balanced equation to represent the acid base reaction
- i. Find the morality of the anhydrated sodium carbonate
ii. Calculate the value of X in the hydrated sodium carbonate.
- a)Describe the use of each of the following during extraction of Sodium
- Calcium Chloride
- Graphite anode
- Steel gauze
b).Why sodium is collected by upwards in the downs cell
c). Write electrodes reaction in downs cell during extraction of Sodium
- a) State Faraday’s Laws of Electrolysis
- Dilute silver nitrate solution was decomposed by the passage of electric current through it. What mass of silver and what volume of oxygen (Measured at s.t.p) would be liberated in electrolysis by 9650 coulombs of Electricity?
- a) In which other areas do we find the warning signs out of laboratory (give four point)
b) Explain how measurements of volume differ when using measuring cylinder and burette
c) It is recommend that laboratory apparatus should be properly washed or wiped after use, explain the significance for this when
- Measuring volume of liquids
- Measuring mass of substance
- a) State three main physical properties of water and show the usefulness of each property
b) State four industrial application of electrolysis
SECTION C (15 MARKS)
Answer one (1) question in this section
- a) How can you tell water is polluted? Give two ways
- We have coal at Kiwira in Mbeya region. Authorities in the government have allowed use of coal for domestic and industrial purpose. What warming can you raise concerning likely effects? Give five points
- a) Define the following terms
- functional group
- homologous series
- isomerism
b) Write down the molecular structure and the IUPAC names of the isomer whose molecular structure is C4H10
c)By naming the reagents, stating the conditions whenever possible using a balances equation describe how ethane could be converted into
- Ethane
- Chloroethane
- 1,2-dibromoethane
- Ethanol
1
FORM FOUR CHEMISTRY EXAM SERIES 68
FORM FOUR CHEMISTRY EXAM SERIES 68
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM FOUR-AUG/SEPT 2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (15 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) 1.4 g of potassium hydroxide is dissolved in water to form 250 cm3 of solution. What is the molarity of this solution?
- 0.01 M
- 0.1 M
- 1.4 M
- 5.6 M
- 6.0 M
(ii) In the blast furnace carbon monoxide is prepared by passing carbon dioxide over a redhot coke. Carbon dioxide is
- an accelerator
- an oxidizing agent
- a reducing agent
- a catalyst
- oxidized.
(iii) Copper can be separated from a mixture of zinc and copper by adding to the mixture
- concentrated H2SO4
- dilute H2SO 4
- aqueous solution of ZnSO4
- concentrated HNO3
- a catalyst.
(iv) 65.25 g sample of CuSO4.5H2O (M = 249.7) was dissolved in water to make 0.800 L of solution. What volume of this solution must be diluted with water to make 1.00 L of 0.100 M CuSO4?
- 3.27 ml
- 383 ml
- 209 ml
- 65.25 ml
- 306 ml.
(v) Which action should be taken immediately after concentrated sulphuric acid spilled on the skin?
- Its should be rinsed off with large quantities of running water.
- It should be neutralized with solid CaCO3
- It should be neutralized with concentrated NaOH.
- The affected area should be wrapped tightly and shown to a medical health provider.
- It should be neutralized with concentrated KOH.
(vi) The only metal which does not react with dilute hydrochloric acid is
- Magnesium
- Aluminum
- Copper
- Zinc
- Sodium.
(vii) 10 cm3 of 0.4 M sodium hydroxide are added to 40 cm3 of 0.2 M hydrochloric acid. The resulting mixture will be
- Neutral
- Alkaline
- Dilute
- Acidic
- Amphoteric
(viii) The ionic equation when aqueous ammonium chloride reacts with sodium hydroxide solution is represented as:
- 2NH+4(aq) + 2Cl-(aq)à 2NH3(g) + Cl2(g) + H2(g)
- NH+4(aq) + OH-(aq) à NH3(g) + H2O(l)
- Na+(aq) + Cl-(aq) à NaCl(g)
- H+(aq) + OH-(aq) à H2O(l)
- 2NH+4(aq) + 2Cl-(aq) à 2NH3(g) + 2HCl(g).
(ix) The apparatus suitable for measuring specific volumes of liquids is called?
- Burette
- Volmetric flask
- Pipette
- Measuring cylinder
- Graduated beaker
(x) The property of metal to be drawn into wires is called?
- Conductivity
- Malleability
- Ductility
- Decorating
- Expansion.
2.Match the physical processes represented by arrows (i) - (v) in List A with the corresponding terms in List B by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. (a) How many chlorine molecules are in 20 cm of chlorine gas at s.t.p? ![]()
(b)Calculate the number of ions present in 5 g of copper II nitrate. (7 marks)
4. A solution contains 40.3g of substance XOH per litre .250.0cm3of this solution required 30.0cm3 of 0.3M sulphuric(VI)acid for complete neutralisation.
(a) Calculate the number of moles of XOH that reacted.
(b) Determine the relative atomic mass of X.
5. Sulphur(IV) oxide is prepared in the laboratory using the set-up in Figure 3.
Study it and answer the questions that follow.
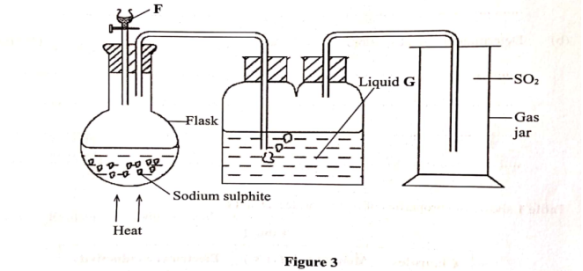
(a) Identify substance F. (1 mark)
(b) Write an equation for the reaction that takes place in the flask. (1 mark)
(c) State the purpose of liquid G. (1 mark)
6. (a) A mass of 1.24g of a divalent metal was deposited when a current of 6A was passed through a solution of a metal sulphate for 12 minutes.Determine the relative atomic mass of the metal( Faraday = 96,500 C mol-1(3 mark)
(b) State two application s of electrolysis.
7. In the Haber process, nitrogen reacts with hydrogen according to the following equation.
3H2(g) + N2(g)→ 2NH3(g) ; AH = -92 kJ/ mol
(a) What would be the effect of adding a catalyst on the position of the equilibrium? (1 mark)
(b) Explain why it is not advisable to use temperatures higher than 773 K in the Haber process. (2 marks)
8. You are provided with solid potassium hydrogen carbonate. Describe how a solid sample of potassium nitrate can be prepared.
9. Study the set-up in Figure 6 and answer the questions that follow.
(a) Name the substance that was collected in tube P. (1 mark)
(b) Write an equation for the reaction which occurs in tube Q in the first few minutes of the experiment. (1 mark)
(c) Give a suitable conclusion for the experiment in the set-up. (1 mark)
10.W is a colourless aqueous solution with the following properties:
I It turns blue litmus paper red.
II On addition of cleaned magnesium ribbon, it gives off a gas that burns with a pop sound.
III On addition of powered sodium carbonate, it gives off a gas which forms a precipitate with calcium hydroxide solution.
IV When warmed with copper(II) oxide powder, a blue solution is obtained but no gas is given of
V On addition of aqueous barium chloride, a white precipitate is obtained.
(a) (i) State what properties (I) and (III) indicate about the nature of W. ( I mark)
(ii) Give the identity of W. (l mark)
(iii) Name the colourless solution formed in (II) and (III). (2 marks)
(iv) Write an ionic equation for the reaction indicated in (V). ( I mark)
11. (a) 20 cm3 of a solution containing 7 g dm-3 of sodium hydroxide were exactly neutralized by 25 cm3 of 0.10 M hydrochloric acid. Calculate the concentration of sodium hydroxide in moles per dm3.
(b) (i) Write the reaction equations involved in the industrial manufacturing of sulphuric acid starting with sulphur dioxide in the contact process.
(ii) Explain why sulphur trioxide is not dissolved directly in water to obtain sulphuric acid in contact process.
12. (a) In the blast furnace, iron ore can be reduced using coke at a temperature of about 1300°C.
(i) Write an equation for the exothermic reaction that causes this high temperature.
(ii) State how carbon monoxide is formed.
(iii) Write a word equation for the formation of slag.
12. (b) Consider elements with atomic number 1, 11, 12 and 17.
(i) What are the types of oxides formed by elements with atomic number 11 and 12?
(ii) Write an equation which represents a reaction between the element with atomic number 1 and 17.
(iii) Write a balanced chemical equation between the oxide of the element with atomic number 11 and aqueous solution of the compound formed in 4 (a) (ii).
SECTION C (15 Marks)
Answer one (1) question in this section.
13. Explain five methods to prevent terrestrial pollution.
14. (a) A current of 0.5 A were made to flow through silver voltameter for 30 minutes. Calculate the mass of silver deposited and the equivalent weight of silver.
(b) Explain the following reactions giving one example in each:
(i) Addition reaction.
(ii)Elimination reaction.
FORM FOUR CHEMISTRY EXAM SERIES 58
FORM FOUR CHEMISTRY EXAM SERIES 58
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRYTERMINALEXAMINATION
FORM FOUR-2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) Which one of the following sets of laboratory apparatus are used for measure volume?
- Crucible, U-tube and volumetric flask
- Test tubes, beakers and glass jar
- Thistle funnel, separating funnel and beaker
- Burette, pipette and measuring cylinder
- Conical flask, test tube and measuring cylinder.
(ii) The empirical formula of certain compound in CH3. Its molar mass is 30 g. What will be its molecular formular?
- CH4
- C2H4
- C2H6
- C2H8
- C4H12
(iii) In order to produce the greatest amount of hydrogen in a short time, one gram of magnesium ribbon should react with
- 10 cm3 of 0.5 M sulphuric acid
- 40 cm3 of 0.5 M acetic acid solution
- 40 cm3 of 0.5 M sulphuric acid solution
- 20 cm3 of 1 M sulphuric acid solution
- 20 cm3 of 1 M acetic acid solution.
(iv) Fractional distillation process of a mixture of water and ethanol is possible because
- water and ethanol have the same boiling point
- water has lower boiling point than ethanol
- ethanol has lower boiling point than water
- water and ethanol form partially immiscible liquid solution
- water and ethanol are immiscible liquids.
(v) Which of the following substances represent a group of acidic oxides?
- Carbon dioxide, carbon monoxide and sulphur dioxide
- Sulphur trioxide, nitrogen dioxide and nnitrogen monoxide
- Carbon dioxide, sulphur dioxide and dinitrogen oxide
- Sulphur trioxide, carbon dioxide and nitrogen dioxide
- Carbon monoxide, nitrogen oxide and sulphur dioxide.
(vi) What will the molarity of a solution which contains 26.5 g of anhydrous sodium carbonate in 5 dm3 of solution?
- 0.05 M
- 0.25 M
- 5.30 M
- 0.025 M
- 0.50 M
(vii) The Brownian movement is taken to be the evidence of the:
- theory of association of water molecules
- theory of ionization of electrolytes
- theory of colloidal suspensions
- kinetic theory of behavior of substances
- Brownian theory.
(viii) One off the isotopes of an element X has an atomic number Z and a mass number A. What is the number of neutrons contained in the nucleus of the element X?
- Z
- A
- A + Z
- A – Z
- Z – A
(ix) C2H4Cl can be represented in different structures which are called
- homologous series
- isomers
- structural formulae
- identical structures
- condensed structures.
(x) _____ is the general term used to explain a mixture of different metals.
- Alloy
- Allotrope
- Amphoteric
- Amorphous
- Isotope.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3.A Form Three student conducted an experiment to prepare a gas in the laboratory by decomposing a certain compound using electricity. She allowed a steady electric current to flow through the solution for 3 hours at s.t.p. If the volume of the gas obtained was 4.12 dm3 and the gas relighted a glowing splint;
(a)name the gas that was produced.
(b)calculate the electric current that was flowing in the solution.
4. (a)An atom M has an atomic number 14 and mass number 28.
(i)What is the number of protons and neutrons?
(ii) Write the electronic configuration of atom M.
(b) Calculate the volume of water which was produced when 1,120 cm3 of oxygen at s.t.p. was liberated during the decomposition of hydrogen peroxide. The density of water = 1.0 g/cm3
5. An experiment was carried out to prepare crystals of magnesium sulphate.
Excess magnesium powder was added to l00cm'of dilute sulphuric(VI) acid in a beaker and warmed until no further reaction took place.
The mixture was filtered and the filtrate evaporated to saturation, then left to cool for crystals to form.
(a) (i) Write an equation for the reaction.
(ii) Explain why excess magnesium powder was used.
(iii) State how completion of the reaction was determined.
(iv) What is meant by a saturated solution?
(v) Explain why the filtrate was not evaporated to dryness.
6. (a) The diagram in Figure 4 was used to prepare hydrogen chloride gas which was passed over heated iron powder.
(i) Give a pair of reagents that will produce hydrogen chloride gas in flask A.
(ii) Name the substance in flask B.
(iii) State the observation made in the combustion tube.
(iv) Write an equation for the reaction in the combustion tube.
(v) Describe a chemical test for hydrogen chloride gas.
(b) (i) Identify the gas that bums at the jet.
(ii) Explain why the gas in (b) (i) is burned.
(b) Write an equation for the complete combustion of ethane.
(c) Give reasons why excess hydrogen chloride gas is dissolved using the funnel arrangement.
(d) State what will be observed when the reaction in the combustion tube is complete.
7. The set-up in Figure 2 was used to prepare a sample of ethane gas. Study it and answer the questions that follow.
(a) Name B - sodium propanoate/:
(b) Write an equation for the complete combustion of ethane.
(c) State one use of ethane.
8. (a) A sample of water is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of sulphate ions.
(b) State one characteristic of a reaction where equilibrium has been attained.
9. Copper(II) ions react with excess aqueous ammonia to form a complex ion.
(a) (i) Write an equation for the reaction that forms the complex ion.
(ii) Name the complex ion.
(b) Explain why CH4is not acidic while HCl is acidic yet both compounds contain hydrogen.
10. The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
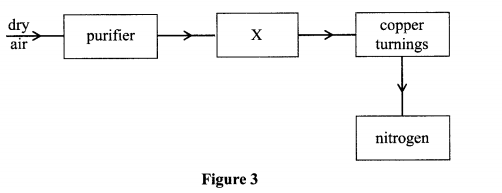
(a) Identify X (I mark)
(b) Write an equation for the reaction with heated copper turnings.
(c) Name an impurity in the sample of nitrogen gas.
11. Study the flow chart in Figure 5 and answer the questions that follow.
(a) Identify substances K and L. K:
(b) Name one reagent that can be used to carry out process J.
FORM FOUR CHEMISTRY EXAM SERIES 48
FORM FOUR CHEMISTRY EXAM SERIES 48
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY 1 MID TERM EXAMINATION
FORM FOUR-2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- Most salts have comparatively high melting points because they have;
- Crystalline structure
- Low pressure
- High specific heat
- Strong electronic attractions between ions
- Strong covalent bond
- A magnesium atom and a magnesium ion have the same;
- Electron configuration
- Number of electrons
- Chemical properties
- Number of protons
- What mass of pure sulphuric acid is found in 400cm3 of its 0.1M?
- 2.45gm B. 9.80gm C. 3.92gm D. 4.90gm
- The volume of 18M concentrated sulphuric acid that must be diluted with distilled water to prepare 10 litres of 0.125M sulphuric acid is;
- 69.44cm3 B. 22500cm3 C. 225cm3 D. 4440cm3
- If two jars labelled W and Z contain 22.4dm3 of oxygen gas and 22.4dm3 of nitrogen gas at STP respectively, then it is true that;
- There were 6.02 x 1023 oxygen molecules in jar W and 6.02 x 1023 nitrogen molecules in jar Z.
- 6.02 x 1023 oxygen atoms were in jar W and 6.02 x 1023 atoms of nitrogen in jar Z.
- There were 12.4 x 1023 molecules of oxygen and nitrogen in the gas jars W and Z.
- 6.02 x 1023 molecules of oxygen and nitrogen were in the two jars W and Z.
- Sodium metal is kept in the oil or kerosene because it;
- Sinks in oil but floats on water
- is very alkaline
- Reacts vigorously with water
- Forms a protective coat of sodium oxide with oil
- The following is one of the characteristics properties of non – metals;
- They are electronegative in nature
- They behave as reducing agents
- They form cations by gaining electrons
- They form anion by loss of electrons
- One of the disadvantages of hard water is that is;
- Causes corrosion of water pipes
- Causes increased tooth decay
- Requires more soap for washing
- Contains minerals that are harmful
- When dilute solutions of calcium chloride and sodium carbonate are mixed;
- A white precipitate of sodium chloride is formed
- A white precipitates of calcium carbonate is formed
- A colourless solution of calcium carbonate and sodium chloride are formed
- A mixture of precipitates of sodium chloride and calcium carbonate are formed.
- A solution of sodium carbonate was prepared in order to get a 2M solution. 200cm3 of this solution was used in a titration experiment. The number of moles present in 200cm3 of 2M solution used in the titration will be;
- 4.0 B. 0.04 C. 0.40 D. 0.045
- Match the responses in list B with the word or phrases in list A by writing a letter of the correct response in the table provided below;
2. (a) Match the items from list A with those in list B.
| LIST A | LIST B |
|
|
3. Figure 1 below represents the laboratory preparation of hydrogen chloride gas.
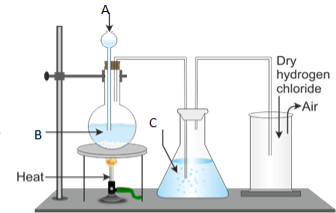
(a) Name the parts labelled A, B, C and D.
(b) (i) Do you think the gas can be collected over water? Give reasons for your answer.
- Explain the test for the gas.
- What is the function of C?
- Name the method used to collect the gas.
- Write a balanced chemical equation for the reaction taking place during the preparation of hydrogen chloride gas.
(c) Write chemical equations for the reaction between:
(i) Ammonia gas and hydrogen chloride.
(ii) Hydrogen chloride gas and water.
4. (a) 1.4gm of potassium hydroxide is dissolved in water to form 250m3 of solution. What is the
molarity of this solution?
(b) What is the molar concentration of a solution containing 1.75 moles of the solute in 3
litres (dm3)?
5. (a) How many molar volumes of 132.0g of CO2 are there at STP?
(b) Determine the number of molecules in 0.25 moles of lead (II) nitrate.
6. (a) What mass in grams of hydrated sodium carbonate (Na2CO3 . 10H2O) in 65cm3 of 0.2M
solution?
(b) What volume of carbon dioxide would be evolved at STP when 6.2g of copper (II)
carbonate is reacted with Suphuric acid?
7. You are given the following symbols of metals’ Zn, Na, Cu, Ag, Mg
- State the metal in each case;
- Which reacts vigorously with cold water?
- Which reacts strongly with steam but not with cold water?
- The metal whose carbonate doesn’t decompose on heating.
- The metal whose nitrate decomposes leaving a metallic residue.
- Write equation for reactions in (a) (i) and (ii).
- Arrange the above metals in order of increasing activity.
8. (a) Give the chemical formula for each of the following;
- Potassium carbonate
- Sodium nitrate (III)
- Iron (III) nitrate
- Aluminum oxide
(b) Complete the following equations and balance them.
- AgNO3(s) heat →
- ZnCO3(s) heat→
- KOH(aq) + H2SO4(aq) →
- CuSO4 . 5H2O(s) heat →
9. (a) What is meant by Dilution?
(b) Determine the litres of water that must be added to 30cm3 of 12M HCl to get a solution
which is exactly 0.25M.
10. (a) State two advantages of hard water.
(b) State two disadvantages of hard water.
(c) Give two methods of softening temporary hardness of water.
11. (a) Define the terms Molecular formula.
(b) Substance X contains 52.2% carbon, 13.0% hydrogen, the rest being oxygen. Calculate the empirical formula of X.
(c) If the density of X is 23, calculate the Molecular formula of X.
SECTION C: 15 MARKS
Answer the questions from this section and include the necessary details.
13. (a) (i) With the aid of a well labelled diagram, explain how you can prepare hydrogen gas
from the laboratory, using zinc metal and dilute hydrochloric acid.
(ii) Write a balanced chemical for the reaction taking place.
(b) (i) What is observed which hydrogen is passed over red hot copper (II) Oxide?
(ii) Write equation for the reaction that takes place in b(i) above.
(c) Which method would you use to prepare big crystals of sodium nitrate in the laboratory?
Explain briefly.
14. 25cm3 of potassium hydroxide were placed in a flask and a few drops of phenolphthalein indicator were added. Dilute hydrochloric acid was added until the indicator changed colour. It was found in the 21cm3 of acid were used.
From above information answer the following questions;
- (i) What piece of apparatus should be used to measure out accurately 25cm3 of sodium
hydroxide solution?
(ii) What colour was the solution in the flask at the start of the titration?
(iii) What colour did it turn when the alkali had been neutralized?
- (i) Was the acid more concentrated or less concentrated than the alkali?
(ii) Name the salt formed in the neutralization.
(iii) Write an equation for the reaction.
(iv) Is the salt, normal or acidic salt? Give reasons for your answer.
FORM FOUR CHEMISTRY EXAM SERIES 46
FORM FOUR CHEMISTRY EXAM SERIES 46
THE PRESIDENTS OFFICE
MINISTRY OF LOCAL GOVERNMENT AND REGIONAL ADMINISTRATION
MOTHLY SERIES EXAMINATIONS
JANUARY 2021 CHEMISTRY FORM 4
- When does a chemist fail to identify a compound between sulphur and
iron?
- a black solid is formed
- heat is used to join them up
- yellow color of sulphur and silvery shinny
- the resulting mass is greater than the individual mass of the elements
- any of the above A-D does not take place.
- Which of the following sets of elements is arranged in order of increasing electro negativity.
A. Chlorine, fluorine, nitrogen, oxygen, carbon
B. Fluorine, chlorine, oxygen, nitrogen, carbon
C. Carbon, nitrogen, oxygen, chlorine, fluorine
D. Nitrogen, oxygen, carbon, fluorine, chlorine
E. Fluorine, nitrogen, oxygen, chlorine, carbon
(iii) The metal nitrate which will NOT give a metal oxide on heating is
- calcium nitrate
- silver nitrate
- lead nitrate
- copper nitrate
- zinc nitrate
(iv) Which of the following pairs of compounds can be used in preparation of calcium sulphate?
- Calcium carbonate and sodium sulphate
- Calcium chloride and ammonium sulphate
- Calcium hydroxide and barium sulphate
- Calcium nitrate and lead (II) sulphate
- Calcium chloride and barium sulphate
(v) Three elements, X, Y and Z, are in the same period of the periodic table. The oxide of X is amphoteric, the oxide of Y is basic and the oxide of Z is acidic. Which of the following shows the elements arranged in order of increasing atomic number?
- X, Y, Z
- Y, Z, Y
- Z, X, Y
- Y, X, Z
- X, Z, Y
- Which action should be taken immediately after concentrated sulphuric acid is spilled on the skin?
- It should be rinsed off with large quantities of running water.
- It should be neutralized with solid CaCO3.
- It should be neutralized with concentrated NaOH.
- The affected area should be wrapped tightly and shown to medical health provider.
- It should be neutralized with concentrated KOH.
- The only metal which does not react with dilute hydrochloric acid is:-
- Magnesium
- Aluminium
- Copper
- Zinc
- Sodium
(viii) Two substances are allotropes of carbon if they:
A. both reduce heated iron (III) oxide to iron
B. have different crystalline structure
D. have equal masses
C. have equal shape
E. have the same arrangement of atoms
(ix) The gas formed when dilute nitric acid reacts with magnesium metal is;
- Nitrogen
- Hydrogen
- Oxygen
- Nitrogen dioxide
- The method of collecting hydrogen chloride gas in a class experiment is known as:
- Downward displacement of water
- Downward displacement of air
- Upward displacement of air
- Fountain
- Condensation
2. Match the following items in list A with those in those in List Bbt writing the correct response beside the item number in answer booklet given.
| LIST A | LIST B |
|
|
SECTION B (70 marks)
3. (a) Differentiate a compound from an element
(b) State the rules used to assign symbols of elements
4.Desribe how you can test for sulphur dioxide gas
(b) What is the effect of presence of sulphur dioxide in the atmosphere?
5. (a) State three commercial uses of water
(b) Explain how we can establish that a given liquid is water.
6. Identify the substances by using the following information:
- A solid is yellow when hot and white when cold.
- When water is added to a white powder heat is evolved and the white powder changes to blue crystals.
- An aqueous solution of a greenish crystalline sulphate forms a pale-green precipitate with sodium hydroxide solution which turns to brown on standing and when exposed to air.
- A colourless gas turns a yellow acidified potassium dichromate paper to green.
- A colourless gas becomes brown on exposure to air.
7. The preparation of ammonia in the laboratory is done by heating a mixture of ammonium chloride and sodium hydroxide.
(a) (i) Write a balanced chemical equation for the above reaction.
(ii) Using balanced chemical equations, state how ammonia reacts with hydrogen chloride gas and heated copper (II) oxide.
(b) (1) State two uses of ammonia.
(ii) Name the catalyst used in the preparation of ammonia.
(c) Explain each of the following reactions, giving observations and equations.
- Aqueous ammonia is added to iron (III) chloride, little by little, until in excess.
- Sodium nitrate is strongly heated.
8. 6. Figure 1 below represents the laboratory preparation of hydrogen chloride gas.
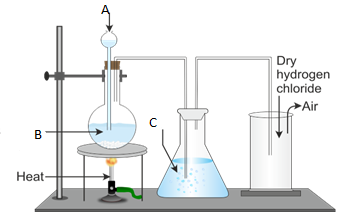
(a) Name the parts labelled A, B, C and D.
(b) (i) Do you think the gas can be collected over water? Give reasons for your answer.
- Explain the test for the gas.
- What is the function of C?
- Name the method used to collect the gas.
- Write a balanced chemical equation for the reaction taking place during the preparation of hydrogen chloride gas.
9.(a)What is fractional distillation?
(b) Give two applications of fractional distillation in the industry.
10.(a) Give four difference between the two carbon allotropes
(b) Give a reason why graphite is used as electrolyte
11. What is displacement reaction?
(b) What will happen when chlorine is bubbled through potassium iodide?
12. (a) Sodium, magnesium, zinc, copper and silver are five metals which appear in this order in the activity series; sodium being the most reactive and silver the least reactive. Which one of these metals is:
- Likely to tarnish most rapidly when exposed to air?
- Most likely to be found free in nature?
- Least likely to react with steam?
- Two of the metals in 12a) above are usually extracted by electrolysis of their molten chlorides. Name the two (2) metals and give one reason of using this method.
SECTION B.(15 MARKS)
Answer only one question.
12. The preparation of ammonia in the laboratory is done by heating a mixture of ammonium chloride and sodium hydroxide.
(a) (i) Write a balanced chemical equation for the above reaction.
(ii) Using balanced chemical equations, state how ammonia reacts with hydrogen chloride gas and heated copper (II) oxide.
(b) (1) State two uses of ammonia.
(ii) Name the catalyst used in the preparation of ammonia.
(c) Explain each of the following reactions, giving observations and equations.
- Aqueous ammonia is added to iron (III) chloride, little by little, until in excess.
- Sodium nitrate is strongly heated.
13. Despite its corrosiveness, sulphuric acid is very important in the industry, Explain six industrial uses of sulphuric acid.
FORM FOUR CHEMISTRY EXAM SERIES 37
FORM FOUR CHEMISTRY EXAM SERIES 37
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
PRE-NATIONAL EXAMINATION SERIES-1
CHEMISTRY FORM-4
2020
TIME: 3:00 HRS
Instructions
- This paper consists of sections A, B and C with a total of fourteen (14) questions.
- Answer all questions in sections A and B and one (1) question from section C.
- Cellular phones and any unauthorised materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet(s).
- The following constants may be used.
Atomic masses: H 1, O- 16, N- 14, S = 32, Zn - 65, Cl -35.5, cu - 64.
Avogadros number= 6.02 x 1023 ![]()
GMV at s.t.p =22.4 dm3 .
1 Faraday= 96,500 coulombs.
Standard pressure = 760 mm Hg. Standard temperature 273 K.
1 litre =1 dm3 =1000 cm 3.
SECTION A (15 Marks)
Answer all questions in this section.
1. For each of the items (i) — (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) "Water is referred to as the universal solvent". What does this mean?
- Water is neither acidic nor basic as compared to other liquids.
- Water exists in three states of matter than any other liquids.
- Water dissolves both organic and inorganic solutes.
- Water is used more domestically than any other liquids.
- Water dissolves more substances than any other known liquids.
(ii) A current of 0.2 A was passed through an electrolyte for 16 minutes and 40 seconds. What is the quantity of electricity produced in coulombs?
- 2000 C
- 1000 C
- 200 C
- 0.20 C
- 7686 C.
(iii) Substance X liberates chlorine gas from acidified potassium chloride. The behaviour of X is described as:
- an oxidising agent
- an oxidising and reducing agent
- catalyst
- a reducing agent
- bleaching agent.
(iv) Which carbonate is the most stable to heat?
- Calcium carbonate
- Copper (II) carbonate
- Lead (II) carbonate
- Zinc carbonate
- .Iron (II) carbonate.
(v) Aluminium does not react with water and does not corrode much in air because
- it is below hydrogen in the reactivity series
- it forms a stable carbonate which prevents reactions
- the metal is covered with a protective coating of an oxide
- aluminium ions have positive charges
- it is very stable.
(vi) Which of the following compounds does NOT belong to the alkenes homologous series?
- C2H4
- C3H6
- C4H 8
- C5H10
- C6H 14.
(vii) Which of the following is NOT among the composition of air?
- Noble gases
- Carbon dioxide
- Nitrogen
- Hydrogen
- Water vapour.
(viii) Chlorine ion, Cl- differs from chlorine atom because it has
- more protons.
- less protons.
- more electrons.
- less electrons.
- more neutrons.
(ix) Which of the following pairs of compounds can be used in the preparation of calcium sulphate?
- Calcium carbonate and sodium sulphate
- Calcium chloride and ammonium sulphate
- Calcium hydroxide and barium sulphate
- Calcium nitrate and lead (II) sulphate
- Calcium chloride and barium sulphate.
(x) Which of the following solutions is the most concentrated?
- 50 g of calcium carbonate in 100 cm3 of water
- 60 g of sodium chloride in 200 cm3 of water
- 65 g of potassium nitrate in 100 cm3 of water
- 120 g of potassium sulphate in 200 cm3 of water
- 50 g of sodium hydroxide in 200 cm3 of water.
2. Match the items in List A which the responses in List B by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. (a) (i) State Avogadro’s law of gaseous volume.
(ii) Find the volume of oxygen gas required to burn completely 1 dm3 of methane. CH4 + 2O 2(g) ? CO 2 + 2H 2O.
(iii) What is the volume of carbon dioxide formed in the reaction at (ii) (4 marks)
(b) Define the following terms:
(i) Mole
(ii) Molecular weight (2 marks)
4. (a) Ammonia gas can be prepared by heating an ammonium salt with an alkali
(i) Name the most common pair of reagents suitable for this reaction.
(ii) Write the equation for the reaction. (4 marks)
(b) Ammonia is very soluble in water and less dense than air. How does each of the properties determine the way in which ammonia is collected in a gas jar?
5. (a) Differentiate empirical formula from molecular formula
(b) Calculate the empirical formula for a compound with the following composition: lead 8.32 g, sulphur 1.28 g, oxygen 2.56 g (relative atomic wt of lead = 207, sulphur = 32, oxygen = 16)
6. (a) Classify the following reactions into oxidation and reduction reactions.
(i) S( s) + O 2( g) ? SO 2( g)
(ii) N2( g) + 3H 2( g) ? 2NH 3( g)
(iii) Fe2+ (aq) e ? Fe3+ (aq)
(iv) Fe3+ (aq) e ? Fe2+ (aq) (4 marks)
(b) What is the oxidation number of iron in iron (III) chloride? (3 marks)
7.(a) Explain the meaning of the following:
(i) Malleable
(ii) Ductile
(iii) Brittle
(b) Give an account of the following
(i) Anhydrous copper (II) sulphate becomes coloured when exposed to the air for a long time.
(ii) Carbon dioxide can be collected by the downward delivery method.
(iii) Concentrated sulphuric acid is not used for drying hydrogen sulphide gas.
(iv) Sodium metal is kept in paraffin oil.
8. (a) (i) What is the first step to take when you want to identify the contents of a given salt containing one anion and one cation?
(ii) In a solution of water, identify a solute and a solvent. Justify your answer.
(b) Sodium is a solid while chlorine is a gas at room temperature although they are in the same period in the periodic table. What is the cause of this difference?
9. (a) (i) Name three gases which should not be produced in order to prevent the destruction of ozone layer.
(ii) List and explain three effects of ozone layer depletion.
(b) Lack of safe water for domestic and industrial uses is a serious problem in most of Tanzanian towns. The major cause of this problem is pollution in the water sources. Slate three methods that could make water from a pond or a well be safe for drinking.
10. (a) (i) Name the products formed when nitrates of potassium and zinc decompose by heat.
(ii) Suggest why the nitrates of zinc and potassium behave differently on heating.
(b) Mention two uses of sodium nitrate.
11. (a) Which ways are the fossil fuels detrimental to the environment? Give four points.
(b) Briefly explain how biogas is produced by using domestic waste.
12. (a) (i) Define isomerism.
(ii) Draw and name two structural formulae of the isomers of C4H8.
(b) Carbon dioxide can be prepared by adding an acid to calcium carbonate.
(i) Using a named acid, write a balanced chemical equation for the reaction. (ii) Name all the products formed in (b) (i)
SECTION C (15 Marks)
Answer one (1) question from this section.
13. Describe the cause, two effects and measures to be undertaken in order to prevent/reduce the amounts of acid rain.
14. 0.48g of a metal, M was placed in a test tube and hot copper (II) sulphate solution was added to it and stirred until the reaction stopped. The metal (M) displaced copper from copper (II) sulphate solution. Copper was filtered, washed with water, dried at 1000 C and the mass found to be 1.27g. Given that, the balanced chemical reaction that occurred is M (s) + CuSO 4(aq)  MSO 4(aq) + Cu (s)
MSO 4(aq) + Cu (s)
(a) Calculate;
- The number of moles of copper that were formed and the number of moles of M that were used in the reaction.
- The relative atomic mass of M and hence identify metal M.
(b) State the appearance of the metal formed (Cu).
(c) With ionic equations, explain why the reaction can be considered to involve both oxidation and reduction.
FORM FOUR CHEMISTRY EXAM SERIES 34
FORM FOUR CHEMISTRY EXAM SERIES 34
THE PRESIDENT�S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- POST-MOCK- EXAMINATION-JUNE
FORM FOUR
Time 3:00 Hours JUNE 2020
INSTRUCTIONS.
- This paper consists of sections A, B and C with a total of fourteen (14) questions.
- Answer all questions in sections A and B and one (1) question from section C.
- Cellular phones and any unauthorised materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet(s).
- The following constants may be used.
Atomic masses: H 1, O- 16, N- 14, S = 32, Zn - 65, Cl -35.5, cu - 64.
Avogadros number= 6.02 x 1023 ![]()
GMV at s.t.p =22.4 dm3 .
1 Faraday= 96,500 coulombs.
Standard pressure = 760 mm Hg. Standard temperature 273 K.
1 litre =1 dm3 =1000 cm 3.
SECTION A: 15 MARKS.
MULTIPLE CHOICE QUESTIONS.
1. For each of the items (i) � (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) When methane undergoes substitutional reaction with excess chlorine, what is the final product?
- Chloromethane
- Dichloromethane
- Trichloromethane
- Tetrachloromethane
- Monochloromethane
(ii) The following are the uses of chromatography except:
- to analyse blood in crime scenes.
- to detect different fibres.
- to detect water pollution.
- to bleach dye/colour.
- to test purity of organic substances.
(iii) Which of the following sets of processes uses a gas that ignites with a "pop" sound when a lighted splint is passed through it?
- Balloon filling, welding and diving
- Hardening oil, balloon filling and welding
- Hardening oil, balloon filling and diving
- Fueling rocket, diving and welding
- Balloon filling, fueling rocket and diving
(v) A current of 0.2 A was passed through an electrolyte for 16 minutes and 40 seconds. What is the quantity of electricity produced in coulombs?
- 2000 C
- 1000 C
- 200 C
- 0.20 C
- 7686 C.
(v) Substance X liberates chlorine gas from acidified potassium chloride. The behaviour of X is described as:
- an oxidising agent
- an oxidising and reducing agent
- catalyst
- a reducing agent
- bleaching agent.
(vi) Which of the following compounds does NOT belong to the alkenes homologous series?
- C2H4
- C3H6
- C4H 8
- C5H10
- C6H 14.
(vii) When a burning fuel produces blue color it means there is
- adequate supply of oxygen with production of soot.
- inadequate supply of oxygen without production of soot.
- inadequate supply of oxygen with production of soot.
- adequate supply of oxygen with production of less heat.
- adequate supply of oxygen with production of more heat.
(viii) Which method could be used to separate the products in the following equation?
![]()
- Chromatography
- Crystallisation
- Distillation
- Filtration
- Condensation.
(ix) Which of the following is NOT among the composition of air?
- Noble gases
- Carbon dioxide
- Nitrogen
- Hydrogen
- Water vapour.
(x) Which of the following pairs of compounds can be used in the preparation of calcium sulphate?
- Calcium carbonate and sodium sulphate
- Calcium chloride and ammonium sulphate
- Calcium hydroxide and barium sulphate
- Calcium nitrate and lead (II) sulphate
- Calcium chloride and barium sulphate.
2. Match the descriptions in List A with the corresponding scientific procedures in List B by writing the letter of the correct response besides the item number in the answer booklet provided.
| List A | List B |
|
|
3. (a) Copper obtained from copper pyrites (CuFeS2) is impure for electrical wiring and has to be purified by electrolysis.
(i) Name the electrolyte and the electrodes used during electrolysis.
(ii) Write the observations that can be made during the electrolysis.
(b) The following flow diagram shows the stages in the contact process
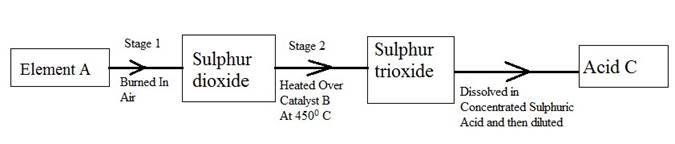
(i) Give the names of element A, catalyst B and an acid C.
(ii) Write a balanced chemical equation for the formation of sulphur trioxide in stage 2
4. (a) Suggest one method of separating each of the following:
(i) Green solution from leaves.
(ii) Alcohol from water.
(b) Elements K, L, M and N have atomic numbers 6, 8, 9 and 20 respectively. Classify each element into its respective period and group.
5. (a) Distinguish manures from fertilizers. Give an example in each case.
(b) The following equation shows the reaction between hydrogen and iodine gas to form hydrogen iodide gas,H2(g) + I2(g) ? 2HI (g) ?H= -800Kj/mol. Giving a reason, explain what would happen to the position of equilibrium if
(i) temperature is lowered.
(ii) hydrogen iodide gas is pumped into the system.
6. (a) State three main physical properties of water and show the usefulness of each property.
(b) State three industrial application of electrolysis.
7. (a)An atom M has an atomic number 14 and mass number 28.
(i)What is the number of protons and neutrons?
(ii) Write the electronic configuration of atom M.
(b) Calculate the volume of water which was produced when 1,120 cm3 of oxygen at s.t.p. was liberated during the decomposition of hydrogen peroxide. The density of water = 1.0 g/cm3
8. (a) Briefly explain why the mixture with equal boiling point cannot be separated by simple fractional distillation.
(b) The preparation of ammonia in the laboratory is done by heating any ammonium salt with an alkali.
(i) Write a balanced chemical equation for the preparation of ammonia gas.
(ii) State two uses of ammonia.
9. (a) Name two elements which are expected to show similar chemical reaction with magnesium. What is the basis for your choice?
(b) State the main raw material and the process involved in the manufacture of the following products.
- Wood charcoal
- Coke
- Lampblack.
10.(a) Give three advantages of using chemical equations over word equations.
(b) You are provided with a compound composed of 22.2% zinc, 11.6% sulphur, 22.3% oxygen, and the rest percentage is water of crystallization. Calculate the molecular formula of the compound if its molecular mass is 283. (7 marks)
11 . A Form Three student conducted experiments in the laboratory to synthesize nitrogen, ammonia and ethane. The experimental results were tabulated as follows:
| Experiment | | Conditions | Products |
| 1 | Lead nitrate | Heat | Lead oxide, oxygen gas and nitrogen gas |
| 2 | Gaseous hydrogen and gaseous nitrogen | Catalyst | Ammonia gas |
| 3 | Ethene gas and hydrogen gas | Catalyst | Ethane |
Write word equations with corresponding chemical equations to summarize the reactions taking place in each of the experiments I to 3. (7 marks)
12.Assume that you are a chemist in a chemical plant that deals with the production of chlorine gas You want to produce 100 litres of chlorine gas per hour so that you can reach the company�s goal of producing 2400 litres every day. What current of electricity will you allow to flow per hour?
13. Describe five causes and effects of soil pollution.
14.Describe four common stages for the extraction of metals. Does the extraction of gold follow all four stages? Give reasons.
Get more of these from www.learninghubtz.co.tz
FORM FOUR CHEMISTRY EXAM SERIES 19
FORM FOUR CHEMISTRY EXAM SERIES 19
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- MOCK- EXAMINATION-MAY
FORM FOUR
Time 3:00 Hours MAY 2020
INSTRUCTIONS.
- This paper consist of three sections A, B and C
- Answer all questions in section A and B and one question in section C.
- The following constants may be used; H= 1. C=12. O= 16, Ag = 108, Cu =63.5
1 Litre = 1dm3 = 1000cm3
G.M.V at STP = 22.4dm3
Avogadro’s constant = 6.02 x 10 23 particles.
SECTION A: 15 MARKS.
- MULTIPLE CHOICE QUESTIONS.
(i) Which among the given list of metals arranged in order of decreasing reactivity with steam from left to right is correct?
- Calcium, Magnesium, Silver, Copper.
- Calcium, Magnesium, Zinc Copper
- Copper, Zinc, Magnesium, Calcium
- Calcium, Zinc, Magnesium Copper.
- Calcium, Magnesium, Zinc, Copper
(ii) A study current of 4A was passed through an aqueous solution of Copper Sulphate for 1800seconds. Mass of Copper deposited will be:
- 63.5
- 31.75g
- 1.185g
- 2.37g
- 11.8g.
(iii) Which of the following statement is true? Avogadro’s Constant is the number of?
- Element in one mole of solid substance
- Atoms in one mole of any gas at S.T.P
- Atoms in one mole of a metal
- Elements needed to liberate one gram of univalent metal
- Elements released when one mole of an element is discharged at the anode.
(iv) An organic compound of structural formula …………………………… belongs to homologous series of:
- Isomers
- Allotropes
- Molecules
- Radicals
- Isotopes.
(v) The atmosphere effect of burning fuel such as wood and petrol oils is to:
- Reduce Oxygen
- Produce clouds
- Add carbon dioxide
- Increase water vapour
- Produce energy.
(vi) Elements loose or gains electrons to form.
- Isotopes
- Radicals
- Molecules
- Ions
- Allotropes.
(vii) Which of the following groups of organic compounds is prepared by dehydration of corresponding alcohol?
- Alkynes
- Alkenes
- Alkanes
- Esters
- Carboxylic acid
(viii) Which of the following is most ductile?
- Alluminium
- Silver
- Copper
- Tin
- Mercury.
(ix) The same current passing through solution of the same concentration of silver nitrate and Copper Sulphate liberates 0.23g of Silver (equivalent weight = 108). The weight of Copper that will be liberated, (equivalent weight 31.8) is?
- 318g
- 0.0677g
- 0.23g
- 0.033g
- 3.18g
(x) In a blast furnace carbon monoxide is prepared by passing carbon dioxide over red – hot coke. Carbon dioxide is:
- An accelerator
- An Oxidizing agent
- A Reducing agent
- A catalyst
- Oxidized.
2. Match the items in List A with responses in List B by writing the letter of correct response beside the item number in List A.
| LIST A | LIST B |
| (i) Isomers (ii) Polymerization (iii) Ethanoic acid (iv) Lubricating oils (v) Homologous series
|
|
SECTION B:
3. (a) Give the reason for use of carbon dioxide
(i) As a fire extinguisher ( ½ marks)
(ii) As a refrigerant ( ½ marks)
(iii) In baking. ( ½ marks)
(b) Explain what will happen when carbon monoxide reacts with:
(i) Oxygen ( 1 marks)
(ii) Concentrated sodium hydroxide ( 1 marks)
(iii) Copper II Oxide. ( 1 marks)
(c) (i) Outline steps in preparation of charcoal ( 11/2 marks)
(ii) Mention two chemical properties of charcoal. (1 marks)
4. (a) Give two example for each or the following
(i) Strong acid ( 1 marks)
(ii) Strong alkali ( 1 marks)
(b) Identify the products formed when strong acid react with
(i) CuO(s) ( 11/2 marks)
(ii) NaOH(aq) ( 11/2 marks)
(c) Explain the meaning of the following and give two examples in each case.
(i) PH scale of an acid ( 1 marks)
(ii) Organic Acid. ( 1 marks)
5. (a) Describe the effect of:
(i) Strongly heating a piece of marble in Bunsen burner flame. ( 11/2 marks)
(ii) Moistening the residue (1) above with water. ( 11/2 marks)
(b)
(i) For what reason is slaked lime added to soil in gardening? ( 2 marks)
(ii) Why is concentrated sulphuric acid used as drying agent? ( 2 marks)
6. The preparation of ammonia M the laboratory is done by heating a mixture of ammonium chloride and sodium hydroxide.
(a)
(i) Write a balanced equation for the reaction ( 11/2 marks)
(ii) Use equations to show how ammonia reacts with hydrogen chloride gas and healed Copper II Oxide. ( 11/2 marks)
(b)
(i) State two uses of ammonia ( 1marks)
(ii) Name the catalyst used in preparation of ammonia ( 1marks)
(c) Explain each of the following reactions giving observation and equations.
(i) Aqueous ammonia is added to iron (III) Chloride, little by little until in excess. ( 1marks)
(ii) Sodium nitrate is strongly heated. ( 1 marks)
7.
a) Draw a well labeled diagram of non – luminous flame of Bunsen burner. 1 marks)
b) Explain the meaning of:
(i) Malleable ( 1/2 marks)
(ii) Ductile ( 1/2 marks)
(iii) Brittle ( 1 marks)
(c) Give an account for the following:
(i) Anhydrous Copper II Sulphate becomes coloured when exposed to air for long time.
( 1 marks)
(ii) Carbon dioxide can be collected by down ward delivery method. ( 1 marks)
(iii) Concentrated sulphuric acid is not used for drying hydrogen sulphide. ( 1 marks)
(iv) Sodium metal is kept in paraffin oil. ( 1 marks)
8.
a) Element A, B, C and D have atomic numbers 6, 8, 17 and 20 respectively. Write electronic structure of those elements ( 2 marks)
b) Write down the formulae of simplest compounds you would expect when;
(i) A and B combine chemically ( 1/2 marks)
(ii) C and D combine chemically ( 1/2 marks)
c)
(i) What types of bonding you would expect between compounds above? ( 1 marks)
(ii) List three differences between bonds you have identified above ( 3 marks)
9. (a)
(i) Name the product formed when nitrate of potassium and Zinc decompose by heating.
( 11/2 marks)
(ii) Suggest why the nitrate of Zinc and potassium behave differently when heating.
( 11/2 marks)
(b) Mention four uses of sodium nitrate. ( 4 marks)
10.
a) Giving four reasons, explain why people who use hard water can expect higher costs than people who use soft water. ( 3marks)
b) Suggest one method for separation of each of the following
(i) Iodine and sand ( 1marks)
(ii) Green solution form leaves ( 1marks)
(iii) Alcohol and water ( 1marks)
(iv) Iron fallings and powdered calcium carbonate. ( 1marks)
11. (a) A current of 0.5A were made to flow through silver voltmeter for 30 minutes. Calculate mass of silver deposited and equivalent weight of silver. ( 2marks)
(b) Explain the following reactions giving one example in each.
(i) Addition reaction ( 2marks)
(ii) Elimination reaction. ( 2marks)
12. (a) With an aid of chemical equations, explain the following terms,
(i) Esterification reaction ( 1marks)
(ii) Substitution reaction ( 1marks)
(iiii) Double decomposition reaction ( 1marks)
(b) Give a reason why alluminium is used in;
(i) Cooking Utensils ( 1marks)
(ii) Overhead Electricals ( 1marks)
(iii) Window Frames ( 1marks)
SECTION C:
Answer Only one Question.
12. Describe the two causes, two effects and measures to be undertaken in order to prevent/reduce the amount of acidic rain. (15 marks)
13. Consider the following.

Use Le Chaletires Principle to describe how the rate of production of D can be altered. (15 marks)
FORM FOUR CHEMISTRY EXAM SERIES 12
FORM FOUR CHEMISTRY EXAM SERIES 12
THE PRESIDENT�S OFFICE
THE MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
MID TERM EXAMINATIONS-MARCH 2020
032 CHEMISTRY- FORM FOUR
Duration: 2:30 Hours
INSTRUCTIONS:-
1.This paper consists of sections A,B and C
2.Answer all questions in all sections
3.Whenever necessary, the following constructs may be used.
Atomic masses: C=12 O=16, H=1, Mg = 24, Na=23, Cl= 35.5, Ca= 40, Cu= 63.5.
Avogadro�s constant = 6.02 x 1023 particles
Molar volume of gas at S.T.P = 22.4dm3mol -1 or 22400cm3 mol -1
SECTION A (15MARKS)
- .Question (i) � (x) are multiple choice items, choose among the given alternatives and write its letter into the answer sheet provided:-
- It compound contain 26.7% carbon, 2.2% hydrogen and the rest is oxygen what is its empirical formula?
- CHO
- C2H2
- CH2O
- CHO2
- The solution are mixed in a beaker and the mass of the beaker and contents is recorded at various times after mixing.
The graph shows the results.
Mass of beaker
And contents
| | |
| | |
Time
The two solutions could be:-
- Aqueous copper (II) sulphate and aqueous ammonia
- Aqueous sodium carbonate and dilute nitric acid
- Aqueous potassium hydroxide and aqueous zinc sulphate
- Aqueous sodium hydroxide and dilute hydro � chloric acid
- A student does an experiment in which three test � tubes containing hydrochloric acid.
The diagram below show the test- tubes containing the experiments. Which metal is placed in each test � tube?
|
| Test tube 1 | Test tube 2 | Test tube 3 |
| A | Iron | Silver | Magnesium |
| B | Iron | Magnesium | Silver |
| C | Magnesium | Silver | Iron |
| D | Silver | Iron | Magnesium |
- A Student decomposes aqueous hydrogen peroxide using manganese (iv) oxide MnO2 as catalyst
The question for the reactions is
2H2O2 Mno2 2H2O+O2
(aq) (l) (g)
- 100 cm3 of hydrogen peroxide is allowed to completely decompose and 120cm3 of oxygen is produced (one mole of a gas occupies 22400cm3 at room temperature and pressure). The concentration of the hydrogen peroxide is :-
- 0.01mol/dm3
- 0.10mol/dm3
- 0.05mol/dm3
- 0.50mol/dm3
- A student was given a sample of a carbonate, M2Co3 where M is a metal. He was asked to find the mass of M2Co3, the mass of M2Co3 and beaker was 7.69g and mass of beaker was 5.99g from this ,the mass of M2Co3 is:-
- 1.71g
- 5.21g
- 7.69g
- 1.70g
- The moles of sodium chloride in 250cm3 of 0.5M sodium chloride are:-
- 0.250Mol
- 0.125Mol
- 2Mol
- 1.25Mol
- Which of the following properties generally increases down the group?
- Ionization energy
- Atomic size
- Electronegativity
- Sodium and zinc
- Which of the following combination is not likely to form covalent bond?
- Magnesium and oxygen
- Nitrogen and oxygen
- Sulphur and fluorine
- Sodium and zinc
- One mole of water corresponds to:-
- 6.02 x 1023 atoms of hydrogen and 6.02 x1023 oxygen atoms
- 22.4dm3 at 1atom and 250c
- 1g
- 18g
- Neutrons are present in all atoms except
- H
- He
- C
- Ne
- Match each item in list A with response in list B by writing its correct letter to the number of corresponding item in the answer sheet(s) provided.
| LIST A | LIST B |
|
|
SECTION B:70 MARKS
Answer ALL questions
- An industrialist has approached you for information on the distillation of crude oil .What advice would you offer as regards the followings:-
- __ Separation of crude oil into fractions
- __ The main fractions of crude oil
- __ Uses of fractions of crude oil
- __ Uses of fractions of crude oil
- __ A schematic representation of the industrial process of fractional distillation of crude oil.
(10 marks)
- (i) Explain the following terms:-
- Standard solution
- The end point of a titration
- 25cm3 of 0.059M sodium hydroxide solution reacted with 23.5cm3 of dibasic acid, H2C2O4. XH2O containing 3.8gdm-3. Given that the ionic equation for the reaction is ;
-Calculate;
i)The molar concentration of the acid
ii)The value of x
d) Write down balanced chemical equation for the reaction involved. (C=12,H=1,0=16)
(10 marks)
- (i) Study the structures below the allotropes of carbon and answer the questions that follows:-
- Identify the allotropes S and R
- Which of the two allotropes is a good conductor of electricity? Explain
- Explain the following
- Carbon dioxide is used as refrigerant
- Carbon dioxide is used as a fire extinguisher (10 marks)
- (i) A weak base containing a few drops of methylorange indicator was titrated with a strong acid and the curve below was obtained.
| | |
| | |
14
pH
12 A
10
8
6 B
4
2
0 Volume of hydrochloric acid added(cm3)
- What will the colour of the indicator at (i) A (ii) B
- Explain why the pH value decreases
- Write down the equations for the reaction, if any, that takes place between dilute hydrochloric acid and each of the following:-
- Copper(II) (b) Lead(II) (c) Zinc (d) Sodium hydroxide (e) calcium hydrogen carbonate.
(10marks)
- A part of periodic table below. The elements are represented by letters which are not the real symbols of the elements.
| 1A |
|
| |||||||
| 3B | 4 | 5 | 6 | 7 | 8C | 9 | 10E | ||
| 11M | 12 | 13X | 14L | 15W | 16H | 17 | 18Z | ||
| 19F | 20 G |
| |||||||
| | | | | | | | | | |
- Write the electronic configuration of the following elements: E,X, L and H.
- Which pair of elements from ions by gaining two electrons?
- Which element is the most reactive metal?
- Which two element when reacted form a liquid which freezes at 00c and boils at 1000c?
- Give the formulae of the oxide and chlorides of elements A,G,X and W.(10marks)
- 7.5g of calcium carbonate was placed in a conical flask containing 50cm3 of dilute hydrochloric acid. The flask was kept at constant temperature and the volume of carbondioxide gas evolved was measured at 20minutes intervals.
- Not all the calcium carbonate was used a during the reaction. The results were recorded in the table below.
| Time from start of reaction (min) | Volume of Co2 evolved(cm3) |
| 0 | 0 |
| 20 | 550 |
| 40 | 810 |
| 60 | 965 |
| 80 | 1000 |
| 100 | 1020 |
| 120 | 1020 |
- Write equation for the reaction between which carbonate and hydrochloric acid
- Plot a graph of volume of carbondioxide (cm3) against time (min)
- What volumes of carbondioxide where evolved during the second 20minutes internal? (20-40)
- Calculate the mass of 11.2cm3 of carbondioxide gas evolved at S.T.P (molar gas volume = 22.4dm3 at S.T.P).
- Determine the mass of calcium carbonate which had reacted after 20minutes. (Ca = 40, O= 16, C= 12) (10marks)
- (a) Explain the changes take place in the solution of concentrated sodium chloride with carbon anode and a mercury cathode.
- Two electrolytic cells for solutions of sodium chloride with carbon and a mercury cathode and aqueous copper (II) sulphate with inert electrodes were connected in series. A current of 1.5A was passed for 600seconds. The first cell contained aqueous sodium chloride with a little sodium hydroxide had copper electrodes and reddish brown precipitate formed.
- Why was there as change in the appearance of the electrolyte in the first cell?
- Why was a small amount of sodium hydroxide added to aqueous sodium chloride in the second cell?
- Name the reddish brown precipitate formed.
- Write an ionic equation for the formation of the substance in (iii)?
- Calculate
- The value for the faraday constant
- The charge on the electrode
- A hydrocarbon has a molecular mass of 56. On combustion 0.28g of hydrocarbon gave 0.88g of carbon dioxide and 0.36g of water
- Calculate the empirical formula of the hydrocarbon
- Draw a structural formula of the hydrocarbon
- To which group of hydrocarbons does the compound belong?
11. The diagram below represents an assembly of the apparatus used to prepare ethene from an alkanol X.
- Name the substances labeled X and Z
- Name substance Y
- Name the conditions under which ethene is prepared from the alkanol X.
10. Zinc metal and hydrochloric acid reacts according to the equation below;
Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g)
3.12g of Zn metal were reacted with 200cm3 of 0.1M hydrochloric acid
(i) Determine the reagent that was in excess
(ii) Calculate the total volume of hydrogen gas liberated at standard temperature and pressure.( Z=65.4, Molar gas volume = 22.4 litres at S.T.P)
12. (a) State Le- chatelier�s principle
(b) The industrial preparation of ammonia in the Haber process is represented by the following equation:
N2(g) + 3H2(g) catalyst 2NH3(g) H= -46.2KJ/mol
| | |
| | |
Study the equation carefully then answer the questions that follow:
What will happen to the position of equilibrium if:
- The temperature of the equilibrium mixture is increased?
- More Nitrogen gas is added to the equilibrium mixture?
- The formed ammonia is removed from the equilibrium mixture?
(c ) What is the use of catalyst in the reaction in 10(b) above?
(d) What is the meaning of the negative sign against the value of heat change -46.2KJ/mol in the chemical reaction given in 10(b) above?
(e) Sketch an energy profile diagram against reaction in 10(b) above.
SECTION C: (15 MARKS)
Answer ALL questions
13. a)Explain what is meant by the following terms:
i) A homologous series
ii) Unsaturated hydrocarbons
iii) Isomerism
b) Write and name all possible isomers of C5H12
c) Write the structures of the following:
i) 2,3-dimethylbutane
ii) 2,3,4-trimethylpent-2-ene
14. Your village is rich in the raw materials for generation biogas: your DDC seeks advice form you as regards:-
- The raw materials.
- The suitability of sitting the biogas plant in the village
- The physical and chemical principle involved
- Economic importance of biogas.
FORM FOUR CHEMISTRY EXAM SERIES 8
FORM FOUR CHEMISTRY EXAM SERIES 8
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







