PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
MID-TERM EXAMS – AUG/SEPT – 2024
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A
Answer ALL questions.
i. Which is not an importance of studying chemistry;
- Understand how matter undergo chemical change
- Make various medical products
- Understand the use of chemicals in agriculture
- Help us enter into various careers.
ii. A mother at home can apply chemistry to;
- Weed flowers in the garden
- Dusting the floor of her house
- Weighing maize flour
- Cooking food in the kitchen
iii. What real takes place during sacrificial protection of iron from rusting?
- The reactive metal remains uncorroded
- The reactive metal is corroded
- The reactive metal and iron are corroded
- The iron is corroded
iv. Which of the following warning signs is likely to appear on a bottle containing concentrated nitric acid in the laboratory?
- Corrosive
- Explosive
- Irritant
- Flammable
v. What is the best definition of Brownian motion?
- Uniform movement of particles
- A motion discovered by Robert Brownian
- A random movement of particles of gas and liquid
- A random movement of particles of gas and solid
vi. What is usually found in the chimney of a Bunsen burner when it is on use
- Oxygen
- The fuel gas
- A mixture of oxygen and the flammable
- Non of the above
vii. A Bunsen burner flame will produce luminous flame when;
- Gas tap is fully opened
- Air holes of Bunsen burner is fully closed
- Air holes of Bunsen burner is fully opened
- Sufficient gas is supplied to the burner
viii. A metal that is usually in liquid form is called;
- Mercury
- B. Bromine
- C. Iron
- D. Sulphur
ix. A symbol which represents the substances which can catch fire easily is;
- Flammable
- B. Toxic
- C. Explosive
- D. Radioactive
x. When anhydrous copper(II) sulphate is left on the glass turns blue. This confirms presence of ………………………. in air;
- Carbon dioxide
- Dust particles
- Water vapour
- Noble gases
- Write the letter of the best match from column B against a name of the element in column A.
| COLUMN A | COLUMN B |
|
|
SECTION B
- (a) What is the aim of using warning sign on containers in the chemistry laboratory?
(b) Name one example of a protective symbol
- Write name of one chemicals under each of the following
- Oxidant/oxidizing agent
- Toxic/poison
- Harmful/ irritant
4. (a) What is a flame?
(b) Write five differences between a luminous flame and a non luminous flame
(c) Draw a well labeled diagram of a luminous flame
(d) Draw a well labeled diagram of a non luminous flame
(e) Mention two advantages of a Bunsen burner over other sources of heat in the laboratory
5. (a) Give the reason to why the chemicals in the laboratory should be labelled and well closed after use.
(b) The shelves in the laboratory should be labelled and constructed in a way that it is at the eye level and not above the eye level. Give the reason behind the statement.
6. Define the following term;
- Air
- Saturated solution
- Unsaturated solution
- Super – saturated solution
- (a) What is distillation
7. (a)mention three branches of science
b) Who is a chemist?
c) List 5 places where chemistry is applied
d) Name three cleaning agents made by chemistry knowledge
8. a) Define the term class B fire.
b) i) Mention two combustible materials in class B fire
ii) Why is water not used to put off oily fires?
iii) Your friends clothes have caught fire. In order to extinguish the fire, you have decided to cover her with a blanket. What is the function of the damp blanket?
c) i) Why is air a mixture and not a compound?
ii) Why is rusting of iron a chemical change?
9. a) Briefly explain the following,
i) Iron left outside for some time will have a brown colour.
ii) Things made of iron rust faster at the coast than at upcountry areas.
iii) When carbon dioxide is passed through lime water for some times, the limewater turns milky.
SECTION C
10. (a) What are the effect if on to fails to follow the following laboratory instructions;
- Failure to follow instructions guided.
- Improper disposal of broken glass materials ……………………………………………………………………………………………….
(d) Write the functions of the following laboratory tools 2 functions;
Round bottomed flask
i. …………………………………………………………………………………………..
ii. …………………………………………………………………………………………..
Burette
i. ……………………………………………………………………………………….
ii. ……………………………………………………………………………………….
FORM ONE CHEMISTRY EXAM SERIES 178
FORM ONE CHEMISTRY EXAM SERIES 178
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
MID-TERM EXAMS – AUGUST – 2023
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A
- For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
- When a chemistry studies a substance, he/she is interested in its
- Force of Attraction
- Shape
- Smell
- properties
- Chemistry is one of the science which deals with,
- alkalinity and Basiaty of substance
- the study of cells
- composition, properties and behavior of matter
- chemical changes
- Which of the following illustrates a chemical reaction taking place in our body
- Falling sick
- Digestion
- Respiration
- Salvation
- Monica found a bottle with chemical which has a source of Oxygen, what warning sign is vicety to be found on it?
- Flammable
- Explosive
- Oxidizing
- Corrosive
- Which best idea can you have about Uranium, Radon, and carbon – 14
- Neither radioactive nor carcinogenic materials
- Radioactive and carcinogenic materials
- Radioactive but not carcinogenic materials
- Non-radioactive carcinogenic material
- Kipps apparatus in used in laboratory for
- Obtaining continuous supply of gas
- Separation of gases
- Drying gases
- Obtaining Pure gases
- A solution is formed when
- A solvent is dissolved in a solute
- A solute is dissolved in a solvent
- A mixture is dissolved in a solvent
- Water dissolved crystals
- Point out the odd mom out in the following groups of elements
- Zinc, sulphur, sodium
- Copper sodium, Iron
- Alluminium, sodium, Zinc
- Sodium, zinc copper
- Separation of the constituents of a mixture by fractional distillation is possible when constituents in the mixture differ in their
- Boiling point
- Solubility
- Melting point
- Freezing point
- The process used to separate a mixture of salt and water is
- Evaporation
- Filtration
- Simple distillation
- sublimation
- Match each item in LIST A with a correct response in LIST B by writing its letter below the number of the corresponding item in table provided.
| LIST A | LIST B |
|
|
SECTION B (70 MARKS)
Answer all questions from this section
- (a)The material resulting from condensation is called condensate. Derive process resulting from following material
- Distillate______
- Filtrate ____
- Sublimate _____
(b)Write the proper method of separating mixtures against each of the following substances
- Oil in seed
- Sand mixed with iodine crystal
- Alcohol mixed with water
- Mixture of salt and milk
- (a) Give best term that fits description below,
- John gave a tentative explanation of why iron rusts when exposed in air
- Mary followed, all steps required in carrying out scientific investigation
- Juma used a region of burning gas to heat in laboratory
(b)What benefits can Salma gain by studying chemistry? Give four
- (a)Write two examples of each of the following
- Heterogeneous mixture
- Homogeneous mixture
- Compound
(b)State the method used to separate the following mixtures and retain their components
- Muddy water
- Common salt
- Oil and water
- Ink
- (a)Supply the suitable word in space provided
- _______ is a mixture of pure metal with another element
- Brass is made by mixing __________ and _______
- Steel in made by mixing ___________ and ___________
- A steel with high content of iron is called _________
- Liquid which mix together completely are called __________
(b) Define the following terms
- Air
- Saturated solution
- Unsaturated solution
- (a)Write chemical symbols of the following chemical names of elements
- Copper
- Sodium
- Zinc
- Aluminum
- Iron
(b)Write chemical names of the following chemical symbols of elements
- S ________
- H ________
- O _______
- N _______
- Cl _______
- (a)Write five differences between luminous and Non-Luminous flame
(b)State advantages of Bunsen burner as sources of heat
- (a)Explain the following
- Laboratory doors should open outwards
- Laboratory should have large windows and doors
- Laboratory should have fume chambers
- Laboratory should have water supply system
(b)State two causes of accident in laboratory
- (a)Define the following terms
(i) First Aid (ii) First Aid Kit
(b) Each student required to learns the basics of first aid for helping accident victims. What are the importances of helping accident victims? Four reasons
(c)Shock is a condition in which the body system is unable to take enough blood to the vital Organs Explain the procedures to follow in giving help for a shock victim
FORM ONE CHEMISTRY EXAM SERIES 138
FORM ONE CHEMISTRY EXAM SERIES 138
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM TWO NEW NECTA FORMAT
FORM ONE EXAMINATION – SEPTEMBER 2022
032 CHEMISTRY
TIME: 2:30 HOURS
INSTRUCTIONS
- This paper consists of sections A and B with a total of TEN (10) questions
- Answer all questions in the space provided.
- All writing must be in black or blue ink except for diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Zn= 65, X =32, O=16
SECTION A (15 Marks)
Answer all questions in this section
- For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the bracket provided.
- The substance that can burn your skin is best described as: -
- Corrosive
- Explosive
- Flammable
- Toxic
- Oxidant
- When burette is used in the laboratory its calibration starts from above because.
- It measures the volume of remained
- It measures the used volume
- It measures the volume added
- It measures the volume of base added
- When burning a fuel produces blue color it means there is
- Adequate supply of oxygen with production of soot
- In adequate supply of oxygen with production of soot
- Adequate supply of oxygen with production of less heat
- Adequate supply of oxygen with production of more heat
- One of the following apparatus is used to measure fixed volume of liquid
- Burette
- Pipette
- Conical flask
- Measuring cylinder
- Volumetric flask
- What should be done if the results obtained from an experiment do not support the hypothesis?
- The results should be left out
- A new problem should be identified
- The experiment should be changed
- Ideas for further testing to find a solution should be given
- The hypothesis should be accepted
- Water exists in three forms, which are solid, liquid and vapour. Which among of the following are examples of liquid form of water
- Rain, snow and hail
- Dew, rain and ice
- Mist, steam and clouds
- Rain, hail, ice
- Rain, mist, and dew
- Emulsion is the mixture of liquids that do not completely mix with each other.
Which of the following set represent emulsion?
- Butter, lotion, toothpaste and milk
- Milk, gel, foam, sponge
- Cream, gel, lotion and paint
- Sponge, milk, smoke, insecticides
- Smoke, milk, paint and body spray
- Domestic utensils made up of iron do rust as a result of the presence of
- Air and fire
- Water and oil
- Air and water
- Air and oil
- Water and fire
- When Mr Mwendakwao took Glucose and dissolved in water to form a uniform mixture.
- water is solution, glucose is solvent and the product is solute
- water is solute, glucose is solvent and the product is solution
- water is solvent, glucose is solute and the product is solution
- water and glucose forms immiscible mixture
- water and glucose form emulsion
- A simple proof that some chemical reactions take place in our bodies is that
A. We eat a balanced diet
B. Doctors tell us so in the hospitals
C. We occasionally fall sick
D. The food we eat or the drinks we take are quite different from the waste products from our bodies
E. There is no proof
2. Match the items in List A with the responses in List B by writing the letter of the correct response beside the item number in the answer sheet or booklet provided.
| List A | List B |
| A. Water is used for extinguishing B. Oxygen and water/moisture C. Presence of Oxygen D. Fire triangle E. Presence of carbon dioxide F. Rusting G. Galvanization H. Class B fire I. Nitrogen |
SECTION B (70 MARKS)
ANSWER ALL QUESTIONS
3. (a) Consider the diagram below;
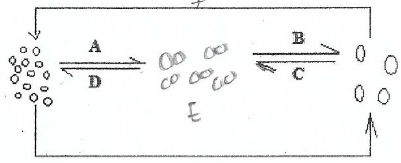
(i) Give aim of the above process
(ii) Identify the process A to F
(iii) Give two importance of the above diagram to our daily life.
(b) State whether the following is permanent change or temporary change,
(i) Dissolution of salt in water
(ii) Rotting of mangoes
4. (a) In which other areas do we find the warning signs out of laboratory (give four point)
(b) Explain how measurements of volume differ when using measuring cylinder and burette
(c) It is recommend that laboratory apparatus should be properly washed or wiped after use, explain the significance for this when
- Measuring volume of liquids
- Measuring mass of substance
5(a) Fill the blanks
- The techniques used to separate serum from blood samples is called _________________________
- The insoluble substances remain in a filter paper during filtration are termed as ___________________________
- Boiling points of substance reflect the strength of __________________
- The sub atomic particles of an atom are ____________________ and _____________________
- Solar energy is example of _______________________resources
(b) Suggest the best method of separating the following mixture
- Alcohol and water
- Sodium chloride and water
- Green solution from leaves
- Sand from rice
- Iron fillings and powder calcium carbonate
6. (a) Mrs. Janeth bought all necessary building materials as he wanted to build modern and expensive house for his mother, four months later he found out that almost all nails and Iron sheets have been rusted as she ignored leakage and small holes in the storage room, unfortunately he has no extra money to buy new nails and Iron sheets. As a chemistry student what will you advice Mrs. Janeth?
(b) Suppose you are in a bus traveling from Moshito Rombo for annual holiday, a bus driver went to the petrol station to fill fuel but he forgets to switch off the bus engine, this caused explosion and fire started. As a form four students:
(i) Will you be able to use fire extinguisher to fight against fire?
(ii) Which type of extinguisher will be suitable for you to use? Give reason of your choice
7. (a) Assign each of the properties to either luminous or non-luminous flame by putting a tick (?) on the respective column in the following table.
| Property of Flame | Luminous Flame | Non- luminous Flame |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b) Assume that you are doing an experiment in the laboratory at 07.30 pm and suddenly the lights go off. Give two reasons to justify the fact that you would consider luminous flame rather than non-luminous flame as an alternative source for lighting.
(c) Identify two properties of the flame produced by the Bunsen burner (air holes full opened) that can not be found in the flame produced by the spirit burner.
8. (a)Giving a reason, state whether rust will form or not in each of the situations (i) - (vi).
- Iron bar is dipped into boiling water.
- Painted iron bar is dipped into un-boiled water.
- Iron bar is dipped in un-boiled water.
- Oiled iron bar is left outside the room over nights.
- Alluminium wire is dipped in un-boiled water.
- A dry iron bar is wrapped with cotton wool.
(b)Briefly explain any four methods of preventing rusting.
9. (a)Why is petrol not recommended to be used as fuel in school laboratories? Briefly explain.
(b) Which three heat sources can be used to boil some water in the laboratory instead of the Bunsen burner?
(c) Arrange the following steps for lighting the Bunsen burner in a correct sequence using letter
A to F.
- Turn the collar to close the air hole completely.
- Turn on the gas fully to ensure that plenty of gas is entering the burner.
- Connect the Bunsen burner to the gas mains.
- Adjust the gas tap until the supply of gas is enough for a time.
- Light the gas at the top of the barrel with a lighted matchstick.
- Close the air hole.
SECTION C (15 MARKS)
10.(a) Identify the five accidents which are common in the laboratory and in each explain possible causes and preventive measures to be taken.
(b). Briefly explain the five classes of fires based on the nature of the burning material and the extinguisher required. Give one example for each class.
(c) Differentiate metals from non metals
FORM ONE CHEMISTRY EXAM SERIES 103
FORM ONE CHEMISTRY EXAM SERIES 103
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM ONE- AUG/SEPT 2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
(i) Air entering the Bunsen burner barrel can be controlled by
- metal ring
- air hole
- metal jet
- air ring
(ii) The appropriate extinguisher used to put off fire caused by cooking oil is:
- Water extinguisher
- Carbon extinguisher
- Wet chemical extinguisher
- Dry air extinguisher
(iii) A non-luminous flame is obtained if the hole is:
- fully opened
- partially opened
- closed
- half opened
(iv) Which of the following components can be separated by filtration method?
- Sand and water
- Kerosene and water
- Ethanol and water
- NaCl and water
(v) All domestic utensils made of iron undergo rusting when exposed to
- Air and fire
- Air and oil
- Air and water
- Water and oil
(vi) A change from gaseous state to solid state without passing through a liquid state is called:
- Deposition
- Sublimation
- Condensation
- Solidification
(vii) When a burning fuel produces blue colour it means there is:
A. adequate supply of oxygen with production of soot
B. inadequate supply of oxygen with production of more heat.
C. inadequate supply of oxygen with production of soot.
D. adequate supply of oxygen with production of more heat.
(viii) Why is water a universal solvent?
- It is neither acidic nor basic than any other known liquid.
- It dissolves more substances than any other known liquid.
- It occurs naturally in all the three states of matter than any other liquid.
- It dissolves both organic and inorganic solutes than any other liquid.
(ix) Which of the following gives the correct meaning of air?
- Mixture of Nitrogen, Oxygen and dust particles.
- Mixture of Nitrogen, Oxygen and Carbon dioxide.
- Mixture of Nitrogen, Oxygen and Water vapour
- Homogenous mixture of gases.
(x) Which of the following is the best apparatus for measuring accurately the volume of a given solution?
- Measuring cylinder
- Burette
- Beaker
- Conical flask
2. (a)Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| LIST A | LIST B |
|
|
(b) Answer the given questions by writing the correct answer in the blank spaces provided.
- The smallest particle of matter……………….
- Used to prepare a solution with known concentration…………..
- Preventing rusting by covering with zinc………………………
- The most abundance gas in air…………………..
- Turns lime water milky………………
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) Mariam was preparing food for her family using hot oil in a frying pan. Accidentally the pan tripped over and a huge fire spread over her kitchen floor.
(i)Mention two extinguishers which would be appropriate for putting out the fire.
(ii)Which fire extinguisher would be dangerous to use when trying to put out the fire in (a) above? Give reason. (b) Mention three conditions for a fire to start.
(c) (i) What is combustion?
(ii) Give three areas where combustion is applied.
4. (a) List down four careers that are a result of studying Chemistry. ![]()
![]() The following are possible causes of accidents which can occur in the Chemistry laboratory. State how you can avoid them.
The following are possible causes of accidents which can occur in the Chemistry laboratory. State how you can avoid them.
(i)Poisonous chemicals left in an unlocked cupboard .........
(ii)A student picking up a bottle containing concentrated H2S04 acid by the neck .........
(iii)Concentrated acids stored in the upper most shelf of cupboard
5.(a) Differentiate between:
(i)an atom and an element
(ii)Combustion and rusting
(iii)a solute and a solvent
(iv)a compound and a mixture
(b)Give two applications of chemistry in everyday life
(c)Why most laboratory apparati are made of glass?
6. Identify the five accidents which are common in the laboratory and in each explain possible causes and preventive measures to be taken.
7. (a) Why candles are not suitable for heating in the laboratory? Give two reasons.
(b) Differentiate luminous from non-luminous flame by giving five points.
8. (a) State one use of each of the items (i) – (x) in administering First Aid.
| S/N | Item | Use |
| (i) | Soap | |
| (ii) | Bandage | |
| (iii) | Sterile gauze | |
| (iv) | Iodine tincture | |
| (v) | Petroleum jelly |
(b) Effective use of the four senses of observation is important before a chemist can make conclusion. With four points, show how the senses are used as tools of observation during experimentation by giving one example for each.
(i) ………… ………… ……………
(ii) ………… …………… ………….
(iii) ………… ……………… …… ….
(iv) ………… …………… ……………
9. Briefly explain the five classes of fires based on the nature of the burning material and the extinguisher required. Give one example for each class.
10. Define the following terms:
(i) Brownian motion
(ii) Compound
(b) Identify whether the following is a physical or chemical change:
(i) Cutting aluminium foil into pieces
(ii) Lighting a match .
(c) How can you separate the following mixtures? Briefly explain.
(i)Water and kerosene
(ii)Salt and water
(iii)Ethanol and water
FORM ONE CHEMISTRY EXAM SERIES 64
FORM ONE CHEMISTRY EXAM SERIES 64
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
AUGUST-SEPTEMBEREXAMINATION SERIES
CHEMISTRYFORM-1
2020
2:30 HOURS
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1.For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) What is the best way of keeping a clean test tube after use?
- Keeping it in water
- Keeping it on a test tube holder
- Keeping it in a basin for test tubes
- Keeping it on a test tube rack.
(ii) Which one of the following does not involve the processes of urban water treatment and purification?
- Sedimentation
- Distillation
- Filtration
- Chlorination.
(iii) Why hydrogen gas is not a constituent of air?
- Because of being water soluble
- Because of being denser than air
- Because of being very light
- Because of being highly flammable.
(iv) Which is the suitable alternative heat source to be used in absence of Bunsen Burner?
- Torch and spirit burner
- Torch and kerosene stove
- Kerosene stove and spirit burner
- Firewood and torch.
(v) What happens when substance A reacts with substance B to form a new substance C?
- Substance A and B are said to have formed a solution
- Substance A and B are said to have undergone a physical change.
- Substance A and B are said to have undergone a chemical change.
- Substance A and B are said to have undergone a dissolution.
(vi) Which components make fire triangle?
- Oxygen, fuel and heat
- Oxygen, nitrogen and heat
- Oxygen, fuel and carbon dioxide
- Oxygen, heat and hydrogen.
(vii) Which state is involved when drying wet clothes?
- Liquid to solid
- Solid to gas
- Gas to liquid
- Liquid to gas.
(viii) Why Non – luminous flame is the most applicable flame for heating purposes?
- It is very nosy
- It has no soot.
- It is very hot
- It has air holes open
(ix) Chemistry is a branch of Science which deals with:
- matter in relation to energy.
- matter in relation to decomposition.
- matter composition and its decomposition.
- properties of conservation of matter.
(x) The process of removing solid contaminants from water is known as:
- water decantation.
- water solidification.
- water purification.
- water sedimentation.
2. (a) Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| List A | List B |
| (i)A solvent which dissolves most substances to form solution. (ii)A substance that has no definite shape or size. (iii)A substance that has a fixed shape and volume (iv)A substance whose components can be separated by physical means (v)Homogeneous mixture of two or more substances. |
|
(b) Fill in the blank spaces by using the appropriate terms
(i)Solid particles formed after filtration......................
(ii)Serum is separated from blood samples by employing a technique called………………..
(iii)Boiling points of substances reflect the strength of……………….
(iv)Grinding chalk into a powder involves changing the state of …………………….
(v) The liquid formed after filtration..........................
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) State one use of each of the items (i) – (x) in administering First Aid.
| S/N | Item | Use |
| (i) | Soap | |
| (ii) | Bandage | |
| (iii) | Sterile gauze | |
| (iv) | Iodine tincture | |
| (v) | Petroleum jelly |
(b) Give one function of each of the following apparatus in the chemistry laboratory.
(i) Spatula…………… …………… ……………….
(ii) Gas jar…………… ……………. …… ……….
(iii) Lie – big condenser …………… …… …………
(iv) Motor and pestle…… …………… ……………
(v) Wire gauze… ………… ………… …………….
4. (a) Differentiate hypothesis from analysis.
(b) Effective use of the four senses of observation is important before a chemist can make conclusion. With four points, show how the senses are used as tools of observation during experimentation by giving one example for each.
(i)………… ………… ……………
(ii)………… …………… ………….
(iii)………… ……………… …… ….
(iv)………… …………… ……………
5. What precautions will you take in handling chemicals having the warning signs shown in the table? Give two precautions in each sign.
| S/N | Sign | Relevant Precaution |
| a) |
| (i)………… ……… …………… …. (ii)………… ………… ……………. |
| b) |
| (i)…………… …………… …. (ii)………… ………… …… …. |
| c) |
| (i)…… …………… ……………. (ii)……… …………… …………. |
| d) |
| (i)…………… ……………… ……. (ii)…………… ………… …………. |
| e) | OXIDANT | (i)…………… ……… ……………. (ii)…………… …………… ………. |
6. Briefly explain the five classes of fires based on the nature of the burning material and the extinguisher required. Give one example for each class.
7. Explain three factors which affect the problem being investigated (b) Explain two areas where scientific procedure is applied.
8. Explain each of the following terms:
(i)Burn
(ii) Bruises
(iii)Fainting ..............................................
(b) What are the six procedures which can be used to help a person with severe bleeding on the wound?
9. Give three differences between the following:
(i)Physical changes and chemical changes
(ii)Mixture and compounds
10.(a) Define the following terms as applied in Chemistry:
(i)Flame
(ii)Bunsen burner
(iii)Laboratory
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
FORM ONE CHEMISTRY EXAM SERIES 18
FORM ONE CHEMISTRY EXAM SERIES 18
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256