THE OFFICE OF THE PRESIDENT, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT.
SECONDARY EXAMINATION SERIES
MARCH 2025
CHEMISTRY FORM TWO
TIME: 2:30 HRS
INSTRUCTIONS
- This paper consists of three(3) sections A, B and C with a total of ten (10) questions
- Section A carries fifteen (15) marks, section B seventy (70) marks and section C fifteen (15) marks.
- Answer ALL questions in all sections
- Write your names and stream on the top center in every page of your examination
- ALL answers should appear in this question paper in the space provided.
SECTION A (15 Marks)
(Answer all questions in this section)
1. For each of the items (i)-(x) choose the correct answer from among the given alternative and write its letter besides the item number in the answer booklet provided. (@1mark=10 marks)
(i) Chemistry is one of the sciences which deals with:
- The study of body cells
- Composition,properties and behavior of matter
- Chemical changes
- Physical changes
(ii) The process of chlorination in water treatment aims at:
- Syrup making
- Removing bad smell
- Killing micro-organisms
- Formation of suspension
(iii) The atomic number of an element is the:
- Number of protons
- Mass number
- Number of neutrons
- Number of protons and neutrons
(iv) The substance that can burn your skin is best described as:
- Flammable
- Corrosive
- Toxic
- Explosive
(v) One of the following can distinguish hydrogen gas from other gases:
- It burns and supports combustion
- It is colourless and odorless
- It gives a smell of a rotten egg
- It burns with pop sound.
(vi) The following sentences suggest that air is a mixture, except:
- Its components can be separated physically
- Its composition varies from one place to another
- Its properties depends on individual gases
- Its formation requires absorption or reabsorption of heat.
(vii) The welders prefers to use non luminous flame for their work simply because
- It is available
- it is easy to transport
- produce very hot flame
- can be made by kerosene
(viii) Spatula in the laboratory is used for scoping what types of substances?
- Liquid and gases
- solids and liquids
- Powdery and gases
- Solids and powdery
(ix) There are two particles inside the nucleus which one contributes the net change of that nucleus?
- Dalton
- Protons
- Electrons
- Neutrons
(x) Why oxygen as one of the components of air Is unique?
- It has ability to burn
- it support combustion
- it is diatonic gas
- combine with carbon dioxide
2. (a) Choose a word(s) from list B which matches the statement or phrases in list A and write its letter in the space provided.
| LIST A | LIST B |
| (i) Factors that can be manipulated to get desired results (ii) A factor that is kept constant (iii) Scientist’s best possible answer (iv) Statement of how the results related to hypothesis (v) First step in scientific method of studying a problem |
|
SECTION C (70) MARKS
3. (a) Define the term rust. (2 marks)
(b) Write down the chemical formula of rust. (2 marks)
(c) Briefly explain why iron in salt water rust faster than in fresh water? (3 marks)
(d) List down three disadvantages of rusting process in our daily life. (3 marks)
4. (a) Give out the reason why oxygen gas is normally collected by the method called downward displacement of water. (2 marks)
(b) Briefly explain how you would test the presence of the following gases in air.
- Oxygen . (2 marks)
- Carbon dioxide (2 marks)
(c) With four reasons explain why air is not termed as a compound. (4 marks)
5. The laboratory technician planned to conduct an experiment for the preparation of gas M. He decided to use a pieces of zinc metal and dilute hydrochloric acid.
- Identify gas M
- Mention six apparatus that he can use to prepare the gas M.
- Write the word equation for the laboratory preparation of gas M.
- Describe the properties of gas M which relates with its uses. Give two points.
6. (a) Explain the following terms;
- Physical change
- Chemical change
(b) Student of form two performing two simple experiments concerning changes of matter on two substances, A and B in the laboratory. In experiment number 1, student changes substance “A” from solid to liquid and in experiment number 2, student changes substance “B” by burning it to form ashes.
(i) Provide one example of each substance between A and B
(ii) What are the four differences between the changes of matter occurred in substance “A” and the changes of matter that occurred in substance “B”
7. a) Identify types of change involved in each of the following. i.e. state whether physical or chemical change,
i) Respiration
ii) Sublimation
iii) Combustion
iv) Distillation
b)Write the chemical symbols of the following elements
i) Potassium
ii) Sodium
iii) Mercury
iv) Gold
c) Write the most suitable method of separating the following mixture.
i) Air
ii) Kerosene and water
iij) Iodine and sand
iv) Syrup
8. A compound consists of 27.3% sodium, 1.2% hydrogen, 14.3% carbon a nd oxygen. Its relative atomic mass is 84
i) Calculate its empirical formula
a. Use the answer in 6(i) to find its molecular formula
ii) State the name of the compound
9. a) Elements 40W has 22 neutrons (the letter is not the actual symbols of the element). State the element's:
i) Atomic number
ii) Number of protons
iii) Electronic configuration
iv) Name of element W
b) Element G is in group 7 period 3.
i) Write the atomic number of G
ii) Write the nuclide notation of two isotopes of G
SECTION C (15) MARKS
10. (a) Scientific procedures are steps used by scientists when finding answers to scientific problems. Write the steps which correspond to each of the following.
- Kelvinia was not feeling well. Shewent to see a medical doctor at Malangali Health Center.
- The Doctor asked Kelvinia several questions about how she was feeling.
- The Doctor ordered Kelvinia’s body temperature, blood and urine sample for observation in the laboratory.
- The laboratory technician diagnosed Malaria parasite in Kelvinia’s blood.
- The doctor confirmed that Kelvinia had Malaria and prescribed medicine for her.
(b) Why is scientific procedure important? Give two points
(c) State three areas where scientific procedures are applied
FORM TWO CHEMISTRY EXAM SERIES 191
FORM TWO CHEMISTRY EXAM SERIES 191
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
MID TERM ONE – MARCH-2024
CHEMISTRY FORM TWO
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A (15 Marks)
Answer all questions in this section
- For each of the following items (i-x).Choose the correct answer from given alternatives and writer its letter besides the item number in the box provided.
- What scientific procedure follows after data interpretation
- Observation
- Hypothesis
- Conclusion
- Experimentation
- The teacher was demonstrating experiment by dissolving Sodium Chloride in water until the solute was not dissolving anymore. What solution was forms at the end?
- Saturated
- Unsaturated
- Super saturated
- Suspension
- A large percentage of air is composed of
- Nitrogen
- Nobel bas
- Carbon dioxide
- Oxygen
- How do chemist refer mixture of water and milk
- Emulsion
- Suspension
- Misable solution
- Immisable solution
- We boil drinking water in order to:
- Remove oxygen
- Remove impulses
- Make it tasteless
- To kill micro-organisms
- The best way of prevent rusting of fragile instrument like camera is
- By using silica gel
- By using ethanol
- By galvanization
- By using oil
- The net charge inside nucleus of an atom is contributed by
- Protons
- Neutrons
- Electrons
- All nucleus
- Why is oxygen a unique component of air?
- Support combustion
- It is diatomic gas
- It forms the largest part of the air
- It has large density
- Which condition is necessary for the nails to rust?
- Oxygen and moisture
- Carbon and oxygen
- Carbon dioxide and oxygen
- Oxygen and nitrogen
- Match each item in LIST A with corresponding answer in list B and write letter of correct answer besides the item number
| LIST A | LIST B |
| (i) Inflating weather balloons |
|
| (ii) Manufacturing of Ammonia | |
| (iii) Manufacture or margarine | |
| (iv) Production of Oxy-hydrogen flame | |
| (v) Manufacture of Hydrochloric acid |
SECTION B – 85 marks
- (a) A laboratory technician instructed form two student to dissolve sodium chloride in distilled water. Give two reasons to state whether a mixture or compound was formed.
(b) Which method can be used useful in separating each the following components?
- Pure water from tea
- Oil from mixture of oil and water
- Ethanol from mixture of water and ethanol
- Salt from sea water
(c) Which change of state of matter is applied in the following process?
- Metallurgy
- Drying materials
- (a)Identify type of changes involve in each of the following processes either physical or chemical
- Respiration
- Sublimation
- Combustion
- Distillation
(b) Write the chemical symbols of the following
- Potassium
- Sodium
- Mercury
- Gold
(c) Give examples of apparatus made up of;
- Porcelain
- Glass material
- (a) Madam Jenipher was preparing Mandazi on a frying pan. Accidentally the pan toppled and huge fire spread on the kitchen floor.
- Which fire extinguisher would be suitable for putting off the fire
- Why water is not suitable to put off the fire?
(b) Give reason to support each of the following
- Commodities like handbags and camera for sale are packed with silica gel
- When iron sheet is exposed to wet and air for longtime they turn to reddish brown color.
- If clothes worn by your friend catch fire, cover them with a fire blanket.
- Mr Mwageni decided to prepare gas M in the Laboratory. He used Hydrogen peroxide as one of the reagent to prepare the gas.
- Identify gas M
- Mention all apparatus used to prepare gas M
- Write word equation for preparation of Gas M
- state uses of gas M
- (a) The chemistry teacher wanted to label container in laboratory with correct warning sign. Help him label the following containers
- Rat poison
- Methylated spirit
- Hydrogen peroxide
- Concentrated Suphuric acid
- Cooking gas
(b) Outline the first aid procedures to a person who has fainted
- (a) Define the following
- water
- water cycle
(b) List four importance processes involved in circulation of water
(c) Identify two commercial use of water
- (a) Name two reagents used to prepare Hydrogen gas in Laboratory
(b) Write word equation for reaction above
(c) Describe chemical test for hydrogen
(d) Explain why hydrogen is not used in balloons nowadays
(e) Give two uses of hydrogen
- (a) What is a flame
(b) Differentiate between the two types of flames
(c) What factors do you consider when choosing heat source in the laboratory?
FORM TWO CHEMISTRY EXAM SERIES 165
FORM TWO CHEMISTRY EXAM SERIES 165
PRESIDENT OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSESSMENT
032 CHEMISTRY FORM TWO
MID-TERM EXAMS MARCH – 2023
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in the spaces provided
- All writing must be in black or blue ink except diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- The following atomic masses may be used: H= 1, N= 14, O= 16, S=32, Ca=40.
SECTION A
1. For each of the items (i) – (x) Choose the correct answer from among the given alternatives and write its letter on answer booklet
- Factors in an experiment that can be manipulated to get desired results are called
- Controlled variable
- Manipulated variable
- Dependent variable
- Independent variable
- One of the following apparatus is used to measure fixed volume of a liquid
- Pipette
- Burette
- Measuring cylinder
- Beaker
- A student who gets burnt accidentally in chemistry laboratory; would be given one of the following first Aid
- Antibiotic solution
- Nitric acid
- Petroleum Jelly
- Potassium Permanganate
- What is the name given to the constant temperature of a substance changing its state from liquid to solid
- Melting point
- Boiling point
- Freezing point
- Sublimation point
- Point out the odd man out in the following group of elements
- Zinc, sulphur sodium
- Copper, sodium, iron
- Aluminum, sodium, zinc
- Sodium, zinc, copper
- Jenipher wanted to obtain pure water from dirt water, which process did she use;
- Evaporation and sublimation
- Evaporation and crystallization
- Evaporation and condensation
- Evaporation and decantation
- The choice of source of heat depend on the
- Color of the flame
- Quantity of heat produced
- Substance to be bummed in air
- Types and shape of flame
- When Anna was preparing food, the frying pan got fire. What type of fire extinguishers would you advice her to use.
- Carbon dioxide
- Fire blanket
- Sand
- Water
- An important property of Oxygen that distinguishes it from other gases is that;
- Burns and Support combustion
- Bum but does not support combustion
- Neither burns or support combustions
- Support combustion
- The chemical used to test for the presence of water in a substance is
- Cobalt II Oxide
- Cobalt III Oxide
- Cobalt Chloride
- Copper II Chloride
2. (a)Match the property in List A with the term in List B by writing the letter of correct letter on space provided
| LIST A | LIST B |
|
|
(b)Fill the blank below with correct answer
- Water is Oxide of __________ and ________
- The catalyst used in preparation of Oxygen is ___________
- Why is hydrogen used in filling balloons _____________
- The reagents used to prepare hydrogen in laboratory are __________ and _____
- The process of circulation of water is atmosphere is called ___
SECTION B
3. Care “P” has the following properties; it is lightly flammable readily combiner with other elements readily reacts with other chemical substance and is a strong reducing agent.
- Name the gas “P”
- What is the method used to collect gas “P” in the laboratory? Give reason
- Give 4 uses of gas “P”
4. (a)Write the names of chemical substances used to test the presence of water
(i) _______ and (ii) _____
(b)Write the examples in which water occur are
- Solid
- gas
(c)Name chemical substance used in laboratory preparation of hydrogen gas.
5. (a)Write a word chemical equation to shoo the deco portion of hydrogen peroxide in the presence of manganese (iv)Oxide
(b)Why Oxygen gas is collected over water?
(c)Respiration and burning are similar process in some ways and difference process in other ways. Give two differences between them
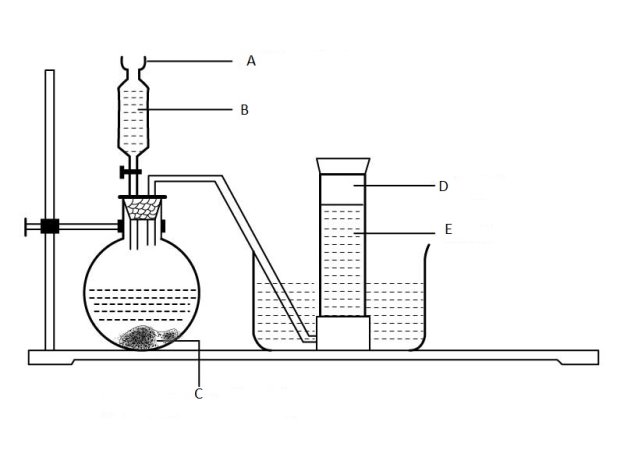
6. Consider the diagram below USED to prepare hydrogen gas and then answer the questions that follow.
|
|
Label parts
- A _________
- B ___________
- C ____________
- D _____________
- F. ___________
7. (a)What is a fuel
(b)Describe six qualities of a good fuel
8. (a)State two importance of studying chemistry
(b)Mention two items used in each of the following categories that are made though application of chemistry
- Agriculture
- Pharmaceuticals
- Household items
- Food and beverage
- Transport
9. (a)State three components of fire Triangle
(b)Mention three conditions for rusting
(c)Rusting occur rapidly in Dar – es – Salaam than in Dodoma Elaborate
10. (a)What is a flame
(b) Write five differences between luminous and non-Luminous flame
FORM TWO CHEMISTRY EXAM SERIES 136
FORM TWO CHEMISTRY EXAM SERIES 136
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM TWO- MARCH/APRIL-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- Write the letter of the best answer in each of the following multiple choice questions. Use the table below.
- What best knowledge can be used by industrialists to manufacture chemicals
- Methods of separating mixtures
- Debate on drug abuse
- Campaigns on elections
- Labeling apparatus for experiments
- How students can protects him against injuries and deaths in a school laboratory?
- To walk very slowly along the corridors of a laboratory
- To study ways of offering first AID
- To observe laboratory rules
- To study uses of products from manufactures
- Which one of the following pair of chemicals is used to swab clean fresh cuts and wounds?
- Methylated spirit and iodine tincture
- Petroleum jelly and copper sulphate
- Milk of magnesia and sodium chloride
- Ethanoic acid and calcium hydroxide
- What is the common use of the following hardware laboratory equipments, a pair of tongs, forceps, clamp and stand, sugar tongs
- To determine mass of the chemicals
- To burn chemicals
- To pick solid chemicals
- To hold objects
- Which is the appropriate warning sign to be pasted on a container that has a chemical which is a source of oxygen
- Flammable
- Explosive
- Oxidizing
- Corrosive
- Which set of apparatus seems to belong in a group of glassware
- Thermometer, crucible and lid, white tile clamp and stand
- Volumetric flask, reagent bottle, beehive shelf, pipe clay triangle
- Separating funnel, burette, pipette, conical flask
- Beakers, cork borer, Bunsen burner, glass rod
- What is the name of the constant temperature of a substance changing its state from liquid to solid?
- Melting point
- Boiling point
- Freezing point
- Sublimation point
- Which process changes a gas directly to solid without the liquid state?
- Condensation
- Sublimation
- Solidification
- Non of the above
- Which of the following process is not a chemical change?
- Soaring of milk
- Burning of paper
- Decomposition of a compound by heating
- Separating mixtures
- Substances which are poor conductors of heat and electricity. What is best idea one can give about the above statement?
- They are metals
- They are non metals
- They are elements
- They are mixtures
- (a) Match the following items by writing the letter against numbers for the best matches. Use the table below
| LIST A | LIST B |
|
|
(b) Fill in the following blanks spaces to make meaningful statement
- …………………………………… is used to separate liquids mixtures of different densities which do not mix. The liquid which comes out first is found at the …………………………………… of the funnel
- A reagent bottle is used to store chemical ……………………………… and the label on the side of the bottle carries the ……………………….of the chemical in the bottle
- Hydrogen gas is a ………………………………… causing substance suck substances are called ……………………………………
- …………………………………… is a small special box which contains items used to offer …………………………………………….
- Detergents are examples of …………………………………pollutants. They interfere with …………………………….balance. B.O.D
SECTION B (80 Marks)
Answer all questions in this section.
- List four (4) chemical properties of oxygen
- Write three (3) common methods of preparations of oxygen in the laboratory
- What is chemical test for oxygen gas?
- List any four (4) uses oxygen gas in our daily life
- Write a word chemical equation to show the decomposition of hydrogen peroxide in the presence of manganese (IV) oxide.
- Why oxygen gas is collected over water?
- Respiration and burning are similar process in some ways and difference process in other ways. Give two differences between them.
10. a) State any four laboratory rules
(b) Consider the diagram below and then answer the questions that follows:
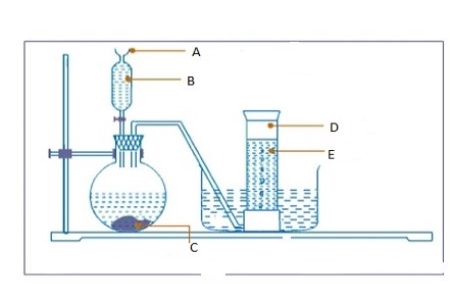
Label parts
- A ………………………………………………………………………………………
- B…………………………………………………………………………………………
- C…………………………………………………………………………………………
- D ………………………………………………………………………………………
- E ……………………………………………………………………………………………..
FORM TWO CHEMISTRY EXAM SERIES 85
FORM TWO CHEMISTRY EXAM SERIES 85
Candidate’s Examination Number ________________________
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY MID TERM EXAMINATION-MARCH
FORM TWO-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(x), choose the correct answer from the alternatives given.
- Isotopes are atoms of the same element that have different
- Atomic number
- Electronic arrangement
- Mass number
- Protons
- The process by which water is converted into water vapour or steam is called
- Condensation
- Evaporation
- Precipitation
- Transpiration
- In the Bunsen burner a sooty flame is most likely to be formed when the
- Air holes are fully closed
- Air holes are opened
- Flame is noisy
- Flame is smaller
- The best method to separate a mixture of iodine and iron is
- Decantation
- Evaporation to dryness
- Fractional to dryness
- Sublimation
- In scientific study, the tentative explanation of the observed chemical phenomenon can be proved by
- Data analysis
- Experimentation
- Hypothesis
- Observation
- The choice of the source of heat depends on the_____
- Colour of the flame
- Quantity of heat produced
- Substance to be burned or boiled
- Type and shape of the flame
- When oxygen combines with metal they:-
- Form metallic oxides
- Form precipitation
- Form rust
- Sublime
- When dilute hydrochloric acid react with metal the product is______
- Hydrogen chloride gas
- Hydrogen gas
- Chloride gas
- Hydrogen peroxide
- Hydrogen react with sulphur to yield_______
- Hydrogen sulphide
- hydrogen sulphate
- Sulphur dioxide
- Sulphur trioxide
- The product smell obtained from the combustion of sulphur in air is_____
- Pleasant
- Toxic
- pungent
- Harmful
| i | ii | Iii | iv | v | Vi | vii | viii | ix | x |
2. (a) You are provided with two list A and list B choose a statement which match the word in list A and write appropriate word in the space provided
| LIST A | LIST B |
|
|
| I | Ii | Iii | Iv | v |
(b) Fill the blanks for each of the following
- _____________________is the smallest particle of an element that has all the chemical properties of that element.
- _______________________is a negative charged particle with a mass of approximately equal to
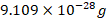 .
. - _________________________is the process of making water clean and safe for human consumption
- __________________________is the process where y aluminium sulphate and impurities in water clump together and sink to the bottom of container
- Water treatment is ________________________________________________________________________________________________________________________________________________
SECTION B (80 Marks)
3. (a) Use a diagram to explain the preparation of hydrogen using dilute hydrochloric acid and zinc granules
(b) Briefly explain four uses of hydrogen
(i)______________________________________________________________________________________________________________________________________________ (ii)_____________________________________________________________________________________________________________________________________________ (iii)_____________________________________________________________________________________________________________________________________________ (iv)_____________________________________________________________________________________________________________________________________________
4. (a) List four Dalton’s atomic theories
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
(b) State four modifications of Dalton’s atomic theories
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
(c) Mention two shortcomings of Dalton’s atomic theory
- ____________________________________________________________________________________________________________________________________
- ____________________________________________________________________________________________________________________________________
5. (a) Write down six physical properties of water
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- __________________________________________________________________
- Give four reasons why water is important in our daily lives and industry.
- ______________________________________________________________________________________________________________________________
- ______________________________________________________________________________________________________________________________
- ______________________________________________________________________________________________________________________________
- ______________________________________________________________________________________________________________________________
6. (a) Give the meaning of each of the following sub atomic particles
- Proton
____________________________________________________________________________________________________________________________________
- Electron
____________________________________________________________________________________________________________________________________
- Neutron
____________________________________________________________________________________________________________________________________
(b) Differentiate between physical and chemical change of matter (five differences)
| Physical change | Chemical change |
| i. | |
| ii. | |
| iii. | |
| iv. | |
| v. |
(c) (i) Write the equation for the preparation of oxygen by heating potassium chlorate
(ii) How oxygen is tested in the laboratory
________________________________________________________________________________________________________________________________________________
7. (a) With chemical equation, give the product of combustion in oxygen of
- A non metal ____________________________________________________________________________________________________________________________________
- A metal , each of your own choice ____________________________________________________________________________________________________________________________________
(b) (i) Define catalyst ________________________________________________________________________________________________________________________________________________
(ii) In experiment of preparation of hydrogen, why copper (II) sulphate is added into the solution of zinc granules and dilute sulphuric acid? ________________________________________________________________________________________________________________________________________________
- Why were the first few bubbles of gas not collected? ____________________________________________________________________________________________________________________________________
- Write the word equation for the reaction during preparation of hydrogen gas by zinc granules __________________________________________________________________
- Suggest two ways how hydrogen is collected during its preparation ____________________________________________________________________________________________________________________________________
- Suggest one problem with collecting hydrogen by upward delivery, remember hydrogen is colourless ____________________________________________________________________________________________________________________________________
8. (a) Explain four methods of preventing rusting of iron (i)______________________________________________________________________ (ii)_____________________________________________________________________ (iii)_____________________________________________________________________ (iv)_____________________________________________________________________
(b) In the following diagram of interconverting of matter name the processes from a to f
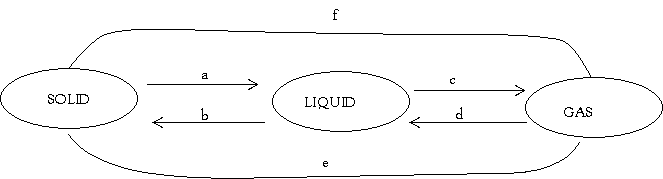
- _________________________________
- _________________________________
- _________________________________
- _________________________________
- _________________________________
- _________________________________
9. (a) Study the diagram below and answer the questions that follow
- What process is taking place? ____________________________________________________________________
- Name the parts labeled A to D
- ______________________________________
- ______________________________________
- ______________________________________
- ______________________________________
(b) For each of the following classes of fire state the burning material and give its suitable fire extinguisher
| Class | Burning material | Fire extinguisher |
| Class A | ||
| Class B | ||
| Class C | ||
| Class D | ||
| Class E |
10. (a) Draw the following warning signs as used in the laboratory, for each give two examples
| Name | Diagram | Examples |
| Toxic | ||
| Flammable | ||
| Oxidant | ||
| Radioactive |
(b) Define each of the following terms
- Warning signs ____________________________________________________________________________________________________________________________________
- Laboratory ____________________________________________________________________________________________________________________________________
FORM TWO CHEMISTRY EXAM SERIES 47
FORM TWO CHEMISTRY EXAM SERIES 47
THE PRESIDENTS OFFICE
THE MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
MID TERM EXAMINATIONS-MARCH 2020
032 CHEMISTRY
Duration: 2:30 Hours
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) (x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) All domestic utensils made of iron undergo rusting when exposed to:
- Air and fire
- Air and oil
- Air and water
- Water and oil
(ii) When a small amount of common salt is dissolved in glass of water the mixture formed is:
- Heterogeneous
- Homogeneous
- Immiscible
- Suspension
(iii) A chemist should acquire all of the following skills except:
- Experimentation
- Observation
- Problem identification
- Surgery
(iv) An important property of oxygen which distinguishes it from other gases is that it:
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor supports combustion
- Supports combustion but does not burn.
(v) The process of chlorination in water treatment aims at:
- Forming suspension
- Killing micro organisms
- Making syrup
- Removing bad odour
(vi) Class E fire can best be extinguished by using:
- Carbon dioxide
- Fire blanket
- Sand
- Water
(vii) The following is a set of apparatuses which are used for heating:
- Crucible, test tube, evaporating dish
- Evaporating dish, tongs, crucible
- Test tube, evaporating dish, tongs
- Tongs, crucible, test tube.
(viii) Which of the following methods can be used to get oil from cotton seeds?
- Decantation
- Distillation
- Grinding and distillation
- Grinding followed by squeezing.
(ix) The substance that can be used to extinguish fire are:
- Carbon dioxide and sand
- Carbon dioxide and sugar
- Nitrogen and sand
- Nitrogen and water
(x) When sugar is dissolved in water, a uniform mixture is formed. The resulting mixture is called a:
- Solute
- Solution
- Solvent
- Suspension
2.(a) Match each item in List A with a response in List B by writing its letter below the number of the corresponding item in the table provided.
| List A | List B |
|
|
(b) Fill in the blank spaces by using the appropriate terms
- In an atom, the effect of the charged nucleons is balanced by the charge of ..
- Serum is separated from blood samples by employing a technique called ..
- Boiling points of substances reflect the strength of .
- Grinding chalk into a powder involves changing the state of .
- The insoluble substances formed during filtration are collectively termed as .
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) Mariam was preparing food foe her family using hot oil in a frying pan. Accidentally the pan tripped over and a huge fire spread over her kitchen floor.
- Mention two extinguishers which would be appropriate for putting out the fire.
- Which fire extinguisher would be dangerous to use when trying to put out the fire in (a) above? Give reason.
(b) Mention three conditions for a fire to start
(c) (i) What is combustion?
(ii) Give three areas where combustion is applied.
4. (a) In an experiment, two iron nails A and B were used whereby painting was applied on nail A. The two nails were placed in a moist environment and after one month the weight of each nail was determined. Which of the two nails would be heavier? Give reason.
(b) State the method which will be used to protect each of the following from rusting:
- Covering iron sheets with a layer of most reactive metals
- Bicycle chain
5. (a) List down four careers that are a result of studying chemistry.
(b) The following are possible causes of accidents which can occur in the Chemistry laboratory. State how you can avoid them.
- Poisonous chemicals left in an unlocked cupboard
- A student picking up a bottle containing concentrated H2SO4 acid by the neck
- Concentrated acids stored in the upper most shelf of cupboard.
6. (a) Mention four physical properties of water
(b) What will happen when?
- A burning splint of wood is introduced into a gas jar containing oxygen gas
- Oxygen gas reacts with metals
- Hydrogen gas reacts with oxygen gas
(c) List four uses of hydrogen in our daily life.
7. (a) Define the following terms as applied in Chemistry.
- Flame
- Bunsen burner
- Laboratory
(b) List four properties of each of the following:
- A luminous flame
- A non luminous flame
(c) Give the use of each of the following components which are found in the First Aid Kit.
- Plaster
- A pair of scissors
- Cotton wool
- Gloves.
8. (a) Define the following terms;
- Chemistry
- Element
- Catalyst
(b) Give three differences between the following:
- Compound and mixture
- Suspension and solution.
9. Gas L has the following properties: it is highly flammable, readily combines with other elements, readily reacts with other chemical substances and is a strong reducing agent.
- Name the gas L
- What is the method used to collect gasL in the laboratory? Give a reason.
- Give four uses of gas L
10. (a) Draw a diagram to show laboratory preparation of oxygen using hydrogen peroxide. In the diagram, label all the compounds and elements involved in the preparation.
(b) Briefly explain how you would distinguish ordinary air from pure oxygen.
(c) List two chemical properties of oxygen gas
FORM TWO CHEMISTRY EXAM SERIES 3
FORM TWO CHEMISTRY EXAM SERIES 3
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256