PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM THREE
ANNUAL EXAMINATIONS – 2023
032/1
Time: 3 Hours
Instructions
- This paper consists of section A, B and C with a total of thirteen (13) questions.
- Answer all questions in this paper
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Atomic masses:
H=1, C=12, O=16, N=14, Ag=108
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4 dm3
1 Faraday = 96,500 coulombs
Standard pressure = 760 mm Hg
Standard temperature = 273 K
1 litre = 1dm3 = 1000cm3
SECTION A. (16 MARKS)
- For each of the items (i) – (x), Choose the correct the answer from among alternatives given and write its letter in the answer sheet provided
- Loose or floppy clothing is not allowed in the laboratory because;
- Movement has to be fast
- It will get wet when water splashes
- It may catch fire or cause one to fall
- It cause poor ventilation in the body
- It prevents experiment from being conducted well
- What mass of Suphuric acid (H2SO4) is found in 400cm3 of its 0.1M?
- 2.67g
- 9.8g
- 4.89g
- 3.92g
- 8.69g
- One of the Isotopes of an element X has atomic number Z and a mass number A. What is the number of Neutron contained in Nucleus of the element?
- Z – A
- A
- A + Z
- Z
- A – Z
- A solution of PH 5 is said to be
- Strong base
- Neutral
- A weak acid
- A strong acid
- A weak base
- In an experiment, 1930 coulombs Liberated 0.64g of copper when the same quantity of electricity. Was passed through a solution of silver Nitrate. What amount of silver was deposited?
- 32g
- 2.16g
- 10.8g
- 108g
- 21.6g
- In blast furnace carbon monoxide in prepared by passing carbon dioxide over a red hot coke. What is the chemical role of carbon dioxide
- An accelerator
- An oxidizing agent
- A catalyst
- A reducing agent
- Flammable
- The following shows four uses of iron, in which of these uses are the iron most likely to rust?
- Iron bucket electroplated with zinc
- Iron wired Alluminium electric cable
- Iron hinges on gate
- Alloyed piston
- Panted iron gate
- Alluminium does not react with water and does not corrode much in air. Why?
- It is below hydrogen in reactivity series
- It forms a stable carbonate which prevent reactions
- The metal in coated with a protective coating of Oxide
- It is very unstable
- Does not react with water
- When bumming fuel produce blue colour, it means there is
- Adequate supply of Oxygen without production of soot
- Inadequate supply of Oxygen without production of soot
- Inadequate supply of Oxygen with production of soot
- Adequate supply of Oxygen with production of more heat
- Inadequate supply of Oxygen with production of more heat
- The reason why white anhydrous copper II sulphate tums blue when exposed in atmosphere is that it.
- Absorb moisture
- React with Oxygen
- React with carbon dioxide
- Becomes dry
- Release water to atmosphere.
- Match items in LIST A with correct response in LIST B by writing the letter of correct response beside the item number in answer sheet provided.
| LIST A | LIST B |
|
|
SECTION B. (54 Marks)
Attempt all questions in this section
- (a)Explain how the following differ from one another
- A base and Alkali
- An atom and isotope
(b) An organic compound P consists of 52.2% carbon 13% hydrogen and 34.8% Oxygen. The vapour density of P is 23. Calculate molecular formula of the compound.
(c) Calculate Oxidation number of Nitrogen in potassium nitrate.
- (a)Give three applications of separation of mixture in our daily life.
(b) 20.0cm3 of sodium hydroxide containing 8.0gdm3 was required for complete neutralization of 0.18g of dibasic acid. Calculate the relative molecular mass of the acid
- (a)State faraday’s Laws of electrolysis
(b) Dilute Silver Nitrate solution was decomposed by passage of electric current through it. What mass of Silver and what volume of Oxygen (measured at (STP) would be liberated in electrolysis by 9650C of electricity?
- 5.3g of X2CO3 was dissolved in water to make 0.5 litre of a solution 25cm3 of this solution required 50cm3 of 0.1M HCl for complete reaction.
- Write a balanced chemical reaction for complete neutralization
- Calculate concentration of X2CO3 in moles dm-3
- What is the relative molecular mass of X2CO3
- Give the name of element X
- (a)What do you understand by the following terms
- Mole concept
- Molar volume of gas
(b)Consider the equation below for dissociation of Suphuric acid
H2SO4(aq) → 2H(aq)+ + SO42-(aq)
From equation, how many ions are there in 9.8g of sulphuric acid?
- (a)Consider the following elements
16O8, 19F9, 20Ne10 23Na11 24Mg12. Atoms and ions of these elements can be 150-electronic (have same number of electrons)
- Write down their symbols when they are ISO-electrode
- Write down their common electronic arrangement in their ions and atoms
(b)Although Sulphur dioxide is an Oxide, It can be further Oxidized
- Write an equation and Condition for further Oxidation of sulphur dioxide
- Write down an equation showing how the product can be used industrially to obtain desired Materials to be produced.
- Give the name for this industrial process
- Write two uses of product formed by industrial process named in (iii) above
SECTION C (30 Marks)
Answer any two questions from this section
- (a)Describe the extraction of sodium from its ore and Write all the reaction equation
(b) State four uses of sodium metal
- (a) Preventing rusting, you should prevent contamination of water and air with iron and steel, also to avoid using material made from iron or steel. State the method that can be used to prevent rusting on each of the following
- Iron sheet
- Bridge and pipes
- Ship
- Machine parts
(b) Fire extinguisher is used to stop fire. List five types of fire extinguishers
- (a)Briefly explain the following observation about a sample of hard water
- When boils it forms white precipitate
- After boiling, water forms a scum
- Sodium carbonate makes the water completely soft
(b) With the acid of chemical equation, briefly describe the following processes
- The removal of temporary hardness of water by boiling
- The removal of permanent hardness of water by chemical means
LEARNINGHUBTZ.CO.TZFORM THREE CHEMISTRY MODAL SERIES 35
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY -SEPT 2022
FORM THREE
032
![]() Time: 3 Hours SEPT, 2022
Time: 3 Hours SEPT, 2022
Instructions
- This paper consists of sections A, B and C with a total of fourteen (14)
questions.
- Answer all questions in sections A and B and one (1)question from section C.
- Sections A and C carry fifteen (15) marks each and section B carries seventy (70) marks.
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your name on every page of your answer sheets.
- The following constants may be used.
Atomic masses: H = 1, O = 16, C = 12, N = 14, Na = 23, Cl = 35.5, K = 39
Ca = 40
Avogadro’s number: ![]()
GMV at s.t.p = ![]()
1 Faraday = 96,500 coulombs.
Standard pressure = 760 mm Hg.
Standard temperature = 273 K.
1 litre = ![]() =
= ![]()
SECTION A (15 Marks)
Answer all questions in this section.
- For each of the items (i) – (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer sheet.
- A Bunsen burner is a chief source of heat in the laboratory that produces both blue and yellow flames. Which one among the following heat sources produce a blue flame:
A: spirit lamp B: gas stove C: kerosene stove
D: candle E: hurricane lamp
- “Water is referred to as the universal solvent.What does this statement mean?
A: it is commonly known liquid
B: it exists in all three states of matter
C: it dissolves more substances than any other known liquid.
D: it is used for cooking, drinking and washing bodies and clothes
E: it is colourless, odourless and tasteless liquid
- Kiraka was suffering from stomach pain for the whole day. Which material among the following could be used to relieve his pain?
A: dilute hydrochloric acid B: cucumber C: lemon
D: tamarind E: blueberries
- Electronegativity increases from left to right across the period in the periodic table. In which group and period does the most electronegative element belong?
A: group I period 3 B: group VII period 2 C: group I period 7
D: group VII period 1E: group VII period 3
- During sunny days, the water in ponds dry completely leaves ponds barely. Which process takes place in that season?
A: condensation B: melting C: evaporation
D: sublimation E: deposition
- The apparatus used to heat small amounts of solid substances within a gas jar is:
A: evaporating dishB: test tube holder C: deflagrating spoon
D: gas jar E: desiccator
- One of the following is a common component that causes reddish brown colour on some materials:
A: sodium metal B: alloy C: water vapour
D: oil E: grease
- Kileo visited the forest at their village, and fortunately, he found some water in the pond mixed with some dust particles. Which simple method among the following he used to get pure drinking water?
A: fractional distillation B: filtration C: condensation
D: crystallization E: simple distillation
- Ammonium ion reacts with sulphate ion to form a compound. The oxidation state of ammonium ion in that compound is:
A: +1 B: 4 C: -1 D: +4 E: -2
- Asha boils the water from the well using electric kettle;but the heating process takes long time. The substance that causes this problem is:
A: aluminium B: calcium C: sodium
D: potassium E: both A and C
- Match the items from list A with the correct responses in list B by writing the letter of the correct response in your answer sheets.
| List A | List B |
| A: Electrovalent compound B: Potassium C:Bromine D: Covalent compound E:Phosphate F:Noble gases G:Metalloids |
SECTION B (70 Marks)
Answer all questions in this section
- Element X found in group II period 4 chemically interacted with element Y found in group VII period 3 forming compound Z.
(a)(i) Write the actual names of element X, Y and compound Z.
(ii) What is the chemical combination involved in this interaction?
(b) (i) Draw the structure of the compound Z
(ii) Give two properties of compound Z.
- (a) Air is a homogeneous mixture of different gases in the atmosphere. Give
three reasons to support this statement.
(b) With the help of balanced chemical equation, explain what will happen to:
(i) A piece of iron bar left to the exposure.
(ii) Anhydrous copper (II) sulphate when put into the watch glass and placed
on the laboratory bench.
- (a) (i) Why chemical symbols are very important to chemist? Give three reasons
(ii) Write the symbols of phosphorous, fluorine, manganese and copper.
(b) Why some elements are assigned symbols with only one letter while
Others bear with two letters?
- A certain compound was found to have the following composition by mass: 24.24% carbon, 4.04% hydrogen and 71.72% chlorine.
- What is the simplest formula of the compound formed?
- Calculate the percentage composition by mass of water in magnesium
Chloride hexahydrate.
- (a) (i) Give two reasons why laboratory exists are advised to open outward?
(ii) Why laboratory safety precaution is very important?
(b) Categorize the following laboratory compounds into corrosive and flammable:
Sodium hydroxide, spirit, sulphuric acid, oil, aro and benzene
- (a) Differentiate molar mass of a substance from molar volume of gases.
- The Golden Boy conducted an experiment for the production of oxygen gas
bythermal decomposition of potassium chlorate. If he used 20g of potassium
chlorate, what volume of oxygen would be produced at s.t.p?
- (a) Briefly state two importance of balancing chemical equations.
- Silver nitrate was introduced into dilute hydrochloric acid to form
products.
- Which type of chemical reaction took place?
- Write the net ionic equation for that reaction.
- (a) Give the names of the processes of making coke from coal and charcoal from
wood.
(b) (i) “Liquid fuel is more advantageous than solid fuel”. Give three points to
support this statement.
(ii) Write down the composition of water gas and producer gas.
- (a) A solution of sodium hydroxide was electrolysed using platinum electrodes.
Write the reactions which take place at the electrodes and give reason why
the solution becomes alkaline.
- What mass of zinc will be formed in electrolysis using a 15 amperes of
electricity for one and a half hours?
- Form three students at Tusomeni Secondary School performed an experiment for the neutralization of sodium hydroxide solution and hydrochloric acid. 25
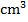 of sodium hydroxide were exactly neutralized by 25
of sodium hydroxide were exactly neutralized by 25 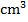 of 0.10M HCl.
of 0.10M HCl.
- Calculate the concentration of sodium hydroxide in:
- mol/
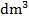 (ii) g/
(ii) g/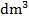
- What is the suitable indicator for:
- The above titration
- The titration of strong acid against strong base
SECTION C (15 Marks)
Answer one (1) question in this section.
- Cutting down trees for firewood and charcoal causes major catastrophic effects to the environment. Using four points analyse these effects and suggest two alternative ways that can be used to minimize the energy loss encountered.
- Water has a property of dissolving some minerals that affect it permanently; as a result, it becomes very disadvantageous to many rural people. As a young chemist and one among these people, explain thefour effects of using this water and suggest two ways of removing the effect.
Page 1 of 5
LEARNINGHUBTZ.CO.TZFORM THREE CHEMISTRY MODAL SERIES 31
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
AUGUST-SEPTEMBER EXAMINATION SERIES
CHEMISTRY FORM-3
2020
TIME: 2:30 HRS
Instructions
- This paper consists of sections A, B and C with a total of fourteen (14) questions.
- Answer all questions in sections A and B and one (1) question from section C.
- Cellular phones and any unauthorised materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet(s).
- The following constants may be used.
Atomic masses: H 1, O- 16, N- 14, S = 32, Zn - 65, Cl -35.5, cu - 64.
Avogadros number= 6.02 x 1023 ![]()
GMV at s.t.p =22.4 dm3 .
1 Faraday= 96,500 coulombs.
Standard pressure = 760 mm Hg. Standard temperature 273 K.
1 litre =1 dm3 =1000 cm 3.
SECTION A (15 Marks)
Answer all questions in this section.
1. For each of the items (i) — (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) "Water is referred to as the universal solvent". What does this mean?
- Water is neither acidic nor basic as compared to other liquids.
- Water exists in three states of matter than any other liquids.
- Water dissolves both organic and inorganic solutes.
- Water is used more domestically than any other liquids.
- Water dissolves more substances than any other known liquids.
(ii) What is the proper set of apparatus would you use to grind granules of a solid substance into fine powder in the laboratory?
- Pestle and filter funnel
- Separating funnel and mortar
- Pestle and filter paper
- Pestle and mortar
- Thistle funnel and mortar
(iii) Which of the following sets of processes uses a gas that ignites with a "pop" sound when a lighted splint is passed through it?
- Balloon filling, welding and diving
- Hardening oil, balloon filling and welding
- Hardening oil, balloon filling and diving
- Fueling rocket, diving and welding
- Balloon filling, fueling rocket and diving
(iv) A current of 0.2 A was passed through an electrolyte for 16 minutes and 40 seconds. What is the quantity of electricity produced in coulombs?
- 2000 C
- 1000 C
- 200 C
- 0.20 C
- 7686 C.
(v) Aluminium does not react with water and does not corrode much in air because
- it is below hydrogen in the reactivity series
- it forms a stable carbonate which prevents reactions
- the metal is covered with a protective coating of an oxide
- aluminium ions have positive charges
- it is very stable.
(vi) When a burning fuel produces blue color it means there is
- adequate supply of oxygen with production of soot.
- inadequate supply of oxygen without production of soot.
- inadequate supply of oxygen with production of soot.
- adequate supply of oxygen with production of less heat.
- adequate supply of oxygen with production of more heat.
(vii) Which of these can be reduced when heated with carbon?
- Aluminium
- Calcium carbonate
- Iron (III) oxide
- Magnesium oxide
- Sodium oxide.
(viii) Which of the following is NOT among the composition of air?
- Noble gases
- Carbon dioxide
- Nitrogen
- Hydrogen
- Water vapour.
(ix) If a steady current of 2 amperes was passed through an aqueous solution of iron (II) sulphate for 15 minutes, the mass of iron deposited at the cathode will be.
- 30g.
- 56g.
- 0.54g.
- 28g.
- 0.52g.
(x) Two substances are allotropes of carbon if
- Both reduce heated iron (II) oxide to iron
- Have different crystalline structure
- Have equal masses
- Have equal shape
- Have the same arrangement of atoms
2. Match the descriptions in List A with the corresponding scientific procedures in List B by writing the letter of the correct response besides the item number in the answer booklet provided.
| LIST 1 | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. (a) How many chlorine molecules are in 20 cm of chlorine gas at s.t.p?
(b)Calculate the number of ions present in 5 g of copper II nitrate.
4. (a) Distinguish temporary hardness from permanent hardness of water.
(b) With the help of chemical equations, explain how you can remove each type of water hardness in 5(a).
5. (a) Copper obtained from copper pyrites (CuFeS2) is impure for electrical wiring and has to be purified by electrolysis.
(i) Name the electrolyte and the electrodes used during electrolysis.
(ii) Write the observations that can be made during the electrolysis.
(b) The following flow diagram shows the stages in the contact process 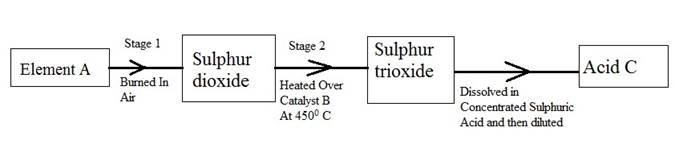
(i) Give the names of element A, catalyst B and an acid C.
(ii) Write a balanced chemical equation for the formation of sulphur trioxide in stage 2
6. (a) Give one example in each of the following:
(i) Alkali earth metals.
(ii) Noblegases .
(iii) Transition elements.
(b) Write the names of the following processes of changing matter from one state to another.
(i) Gas to liquid.
(ii) Ga s to solid.
(iii) Solid t o gas .
7. (a) State four steps employed in the extraction of moderate reactive metals.
(b) Write balanced chemical equations to show how chlorine reacts with the following:
- water.
- aqueous iron (II) chloride solution.
- hydrogen sulphide.
8. (a) State three main physical properties of water and show the usefulness of each property.
(b) State three industrial application of electrolysis.
9. (a)An atom M has an atomic number 14 and mass number 28.
(i)What is the number of protons and neutrons?
(ii) Write the electronic configuration of atom M.
(b) Calculate the volume of water which was produced when 1,120 cm3 of oxygen at s.t.p. was liberated during the decomposition of hydrogen peroxide. The density of water = 1.0 g/cm3
10. (a) Determine the empirical formula of a substance that has the following composition by mass; 49.5% oxygen.
(b) Give one reason why Alluminium is chosen to make each of the following items:
- Cooking foil
- Overhead electric cables
- Window frames
11. (a) Identify and state the environmental problem caused by the gas which is released from the blast furnace in the extraction of iron from its oxide.
(b) (i) Draw a labeled diagram of a simple electrolytic cell which show how copper is purified.
(ii) Write balanced ionic equations to show the electrode reactions which occur when copper is purified.
12. (a) (i) Why chemistry laboratory exits open outward?
(ii) State the uses of any four items found in a First Aid Kit.
(b) (i) Arrange the following metals in order of increasing reactivity; zinc, magnesium, calcium, copper and mercury.
(ii) Which one of the metals in (b) (i) above reacts with steam to form an oxide which is white when cold and yellow when hot?
SECTION C (15 Marks)
Answer one (1) question from this section.
13. In Tanzania, soil conservation is very important for Industrial Materials production. Explain six methods that are used to manage loss of plant nutrients from the soil.
14. 0.48g of a metal, M was placed in a test tube and hot copper (II) sulphate solution was added to it and stirred until the reaction stopped. The metal (M) displaced copper from copper (II) sulphate solution. Copper was filtered, washed with water, dried at 1000 C and the mass found to be 1.27g. Given that, the balanced chemical reaction that occurred is M (s) + CuSO 4(aq)  MSO 4(aq) + Cu (s)
MSO 4(aq) + Cu (s)
(a) Calculate;
- The number of moles of copper that were formed and the number of moles of M that were used in the reaction.
- The relative atomic mass of M and hence identify metal M.
(b) State the appearance of the metal formed (Cu).
(c) With ionic equations, explain why the reaction can be considered to involve both oxidation and reduction.
LEARNINGHUBTZ.CO.TZFORM THREE CHEMISTRY MODAL SERIES 25
MODEL EXAMINATION PAPER 2
CHEMISTRY F3
NAME………………………………….………………..CLASS…………………………….……………TIME: 3HRS
INSTRUCTIONS:-
1. This paper consists of sections A, B and C
2. Answer all questions in all sections
3. Whenever necessary, the following constructs may be used.
- Atomic masses : O= 16, Na= 23, S=32,H=1, K=39, Al= 27,
- GMV at STP = 22.4dm3
- Avogadro’s constant = 6.02 x 1023
- 1litre = 1dm3= 1000cm3.
SECTION A (15 marks)
- Write the letter of the correct answer in the answer sheet (s) provided for each of the following questions (i) to (x).
(i) All domestic utensils made of iron undergo rusting when exposed to:-
- Air and fire
- Air and oil
- Air and water
- Water and oil
(ii) When a small amount of common salt is dissolved in a glass of water the mixture formed is:-
- Heterogeneous
- Homogenous
- Immiscible
- Suspension
(iii) A chemist should acquire all of the following skills EXEPT:-
- Experimentation
- Observation
- Problem identification
- Surgery
(iv) An important property of oxygen which distinguishes it from other gases is that it:-
- Burns and supports combustion
- Burns but does not support combustion
- Neither burns nor support combustion
- Support combustion but does not burn
(v) The process of chlorination in water treatment aims at:-
- Forming suspension
- Killing micro- organisms
- Making syrup
- Removing bad odour
(vi) One of the following is not correct about coke being a better fuel than coal as it:-
- Does not produce carbon dioxide gas
- Does not produce poisonous gas
- Has a higher heat content
- Is clean and smokeless
(vii) Class E fire can best be extinguished by using:-
- Carbon dioxide
- Fire blanket
- Sand
- Water
(viii) The following is a set of apparati which are used for heating:-
- Crucible, test tube, evaporating dish
- Evaporating dish, tongs, crucible
- Test tube, evaporating dish, tongs
- Tongs, crucible, test tube.
(ix) Which of the following methods can be used to get oil from cotton seeds?
- Decantation
- Distillation
- Grinding and distillation
- Grinding followed by squeezing
(x) Which of the following apparati is suitable for measuring volumes of smaller quantities of liquids?
- Beaker
- Burette
- Conical flask
- Measuring cylinder
- Match each item in list A with response in list B by writing its correct letter to the number of corresponding item in the answer sheet(s) provided.
| LIST A | LIST B |
| (i) Chemical equation (ii) Liquid metal (iii) Ammonia (iv) Deliquescent
|
|
SECTION B (54 marks)
- (a) Define the term “neutralization reaction” (give one example)
(b) Write down the names and formulae of three common acids in the laboratory.
(c ) What is an indicator? Give four (4) examples of acid- base indicators.
(d) Write down the products formed when each of following pairs of compounds react:
(i) Acid and metal
(ii) Acid and metal carbonate (10 marks)
- (a) Complete and balance the molecular equations for the following reactions:-
![]() (i) CaCO3(s) + heat
(i) CaCO3(s) + heat
![]() (ii) K2CO3 (s) + HCl (aq)
(ii) K2CO3 (s) + HCl (aq)
![]() (iii) Pb (NO3)2 (aq) + Na2 SO4 (aq)
(iii) Pb (NO3)2 (aq) + Na2 SO4 (aq)
(b) Write a balanced chemical equation and its corresponding ionic equation for the reaction between dilute sulphuric acid and:-
(i) Sodium hydroxide solution.
(ii) Zinc granules.
N.B. show all the state symbols. (10 marks)
- (a) Define the terms (i) Mole (ii) Molar mass
(b) Calculate the molar mass of (i) Al2 (SO4)3 (ii) Na2 Co3 10H2O
( c) State the Avogadro’s law
(d) How many oxygen molecules are there in 20cm3 of oxygen gas at STP?(10 marks)
- (a) (i) Name any four heat sources in the chemistry laboratory
(ii) Name two types of flames produced by the Bunsen burner
(iii)How do the two above mentioned flames differ?
(b) Write down four (4) careers that are a result of studying chemistry.(10 marks)
- The diagram below represents the laboratory preparation of oxygen, study the diagram and then answer the questions which follow:-
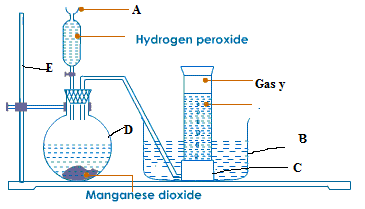
(a) (i) Label the parts indicated with letters A, B, C, D in the diagram
(ii) Does Oxygen burn? Why?
(b) The formula of manganese (iv) oxide is MnO2 and that of hydrogen peroxide is H2O2. Which compound produces oxygen?
(c) (i) What is the name of the method of collection the gas?
(ii) Explain the meaning of catalyst.
(iii)How can you test for oxygen? ( 10 marks)
- Study the following part of the periodic table and then answer the questions that follows.
Note: The letters used are not the scientific symbols for the elements concerned .
|
I O | |||||||
|
| II | III | IV | V | VI | VII |
|
|
|
|
|
|
|
| N |
|
|
| K |
|
|
| Q |
| P |
| L |
|
|
|
|
|
|
|
(A) Identify and write down the electronic configuration for the elements K,N,P and L
(B) What type of bond will exist in a compound formed when Q combines with L? write the chemical formula for the compound formed and list two chemical properties of the compound formed. ( 10 marks)
9. Zinc metal and hydrochloric acid reacts according to the equation below;
![]() Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g)
Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g)
3.12g of Zn metal were reacted with 200cm3 of 0.1M hydrochloric acid
(i) Determine the reagent that was in excess
(ii) Calculate the total volume of hydrogen gas liberated at standard temperature and pressure.( Z=65.4, Molar gas volume = 22.4 litres at S.T.P) (10 marks)
SECTION C (15 marks)
- (a) List six (6) factors which can alter the rate of a chemical reaction.
(b) Copy and complete the passage about the manufacture of sulphuric acid.
Note: Underline your answers.
Passage:-
Sulphuric acid is made by contact process.
First sulphur or sulphur - containing compounds are burned in air to produce ________gas.
This gas is purified to prevent the catalyst being_____________at a later stage.
This gas and air are passed over a _____________catalyst to produce sulphur trioxide.
Sulphur trioxide dissolves in concentrated sulphuric acid to produce_______________.
This is diluted with_______________ to produce an ordinary concentrated sulphuric acid.
- (a) State Le- chatelier’s principle
(b) The industrial preparation of ammonia in the Haber process is represented by the following equation:
![]()
![]()
![]() N2(g) + 3H2(g) catalyst 2NH3(g) H= -46.2KJ/mol
N2(g) + 3H2(g) catalyst 2NH3(g) H= -46.2KJ/mol
![]()
![]()
Study the equation carefully then answer the questions that follow:
What will happen to the position of equilibrium if:
(i) The temperature of the equilibrium mixture is increased?
(ii) More Nitrogen gas is added to the equilibrium mixture?
(iii) The formed ammonia is removed from the equilibrium mixture?
(c ) What is the use of catalyst in the reaction in 10(b) above?
(d) What is the meaning of the negative sign against the value of heat change -46.2KJ/mol in the chemical reaction given in 10(b) above?
(e) Sketch an energy profile diagram against reaction in 10(b) above.
LEARNING HUB.TZ Page 1
LEARNINGHUBTZ.CO.TZFORM THREE CHEMISTRY MODAL SERIES 13
MODEL EXAMINATION PAPERS
CHEMISTRY FORM THREE
MODEL PAPER 1
NAME…………………………………..CLASS…………………………….……………………TIME 3HOURS
INSTRUCTIONS:-
- This paper consists of three sections- A, AND B
- Answer all questions in section A and B.
- This paper consists of section A, B, and C
- Answer all questions
- You may use the following constants: Na = 23, C = 12, O = 16, IGMV = 22.4dm3 IF = 96500C, NA = 6.02 X 1023
SECTION A.
- Write the letter of the best answer in the box provided below
(i) How would you protect yourself from direct contact with corrosive chemical in the lab?
- Use protective clothing
- Avoid smoking
- Never pour or threw into sinks
- Don’t smell the chemical
(ii) What is the use of a funnel?
- To filter solutions
- To lead fluids into flasks
- To drop liquids onto other chemicals
- Multipurpose
(iii) Which part of a blue Bunsen flame is not so hot?
- The pale blue zone
- The green zone
- The tip
- The base
(iv) Which set of compounds have names ending in ide?
- Acids
- Bases
- Binary
- Inorganic
(v) Which of the following is not a homogenous mixture?
- Alloy
- Brine
- Sugar solution
- Muddy water
(vi) Which method is used to separate oxygen from air?
- Distillation
- Fractional distillation
- Filtration
- Evaporation
(vii) What is term used for a substance resulting from condensation process?
- Distillate
- Sublimate
- Liquid
- Condensate
(viii) What is the colour of phenolphthalein in alkali solution?
- Yellow
- Colourless
- Blue
- Pink
(ix) Which substance will change wet litmus paper from blue to red?
- Lemon juice
- Ash in water
- Sodium carbonate
- Sodium chloride
(x) Which substance produce a pH of 14 when tested
- Alkalis
- Acids
- Metals
- Non-metals.
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
SECTION B.
- Match the lettered items to the numbered items
Numbered item:
(i) Normal salt
(ii) Crystallization by boiling
(iii) Nonsense combination of ions for a formula
(iv) Most reactive elements in the periodic table
(v) Another name for inert gases
Lettered items
- Deliquescent
- Hydroscopic
- Preparation of soluble salts
- Preparation of insoluble salts
- A compound containing –ve and +ve ions in solution other than OH or H+ ions
- A compound whose hydrogens are party displaced
- Produce precipitates or gases
- Produce ions
- Permanent hardness of water
- Temporary hardness of water
- Atoms are in a simple ratio of combined masses
- Compound has actual number of atoms combined
- Sodium nitrate
- Sodium chloride
- KNa0
- AlCl3
- Noble gases
- Lithium, potassium
- Fluorine, francium
- Air
- Does not have replaceable hydrogen
- (a) Draw a well labeled diagram of Downs cell and show the half reactions in the extraction of sodium
(b) What is the effect of temperatures above 800°C in process (a) above?
(c) How can the temperature of 800°C be lowered to 600°C?
- Write ionic equations of the following chemical reactions
(a) Aqueous sodium carbonate reacts with dilute hydrochloric acid
(b) Silver nitrate solution reacts with dilute magnesium chloride solution
(c) Zinc metal is put in a solution of copper sulphate
(d) Barium chloride solution reacts with sodium sulphate solution
(e) Calcium metal is reacted with cold water
- (a) What is molecular formula?
(b) Compound Q was found to have the molar mass of 106 with the following percentage composition by mass 43.4% sodium, 11.3% carbon, 45.5% oxygen. Find the molecular formula of the compound Q and write its chemical name.
- Find solutions A – E have the following pH values: A. ph1 B. PH6 C. PH7 D. PH9 E. PH12
(a) Which of the solutions:
(i) Is exactly neutral
(ii) Is strong acidic
(iii) Is weakly alkaline
(b) Could be a solution of carbon dioxide in water
(c) Will give universal indicator colour (i) Green (ii) Red
(d) Will contain a large concentration of OH ions
(e) Could be a solution of ammonia in water?
- (a) Write the IUPAC names of the following compounds
(i) NaClO3
(ii) K1O3
(iii) NaH
(iv) Ca(NO3)
(v) NO
(b) Write the chemical formula of the following compounds
(i) Lead (II) chloride
(ii) Aluminium hydride
(iii) Sodium hydrogen phosphate (v)
(iv) Sulphur oxide (VI)
(v) Ammonium chloride
SECTION C
- (a) State the chaterliers principle
(b) With the help of an energy level diagrm on the reaction
![]() CaCO3 CaO(g) + CO2(g)
CaCO3 CaO(g) + CO2(g)
?H = + 175.5 kJ. Mol -1
(i) Show weather the reaction is exothermic or endothermic
(ii) Write down the type of the reaction shown by the above equation
(c) What will be the effect on the proportion of calcium carbonate in the equilibrium mixture in the above equation if
(i) The temperature is increased?
(ii) The pressure is increased?
- (a) Define electrolysis
(b) An electric current was passed in series through solutions of calcium chloride and copper (II) sulphate using carbon electrodes in the electrolytes. If 2.5dm3 of chlorine gas was collect at stp what volume of oxygen and what mass of copper were formed at the same time?
- Ammonia in industry is manufactured by Haber process
N2(g) + 3H2(g) 2NH3(g) + 46.2 kJ. mol-1
By the help of a well labeled diagram explain how the above process takes place under the influence of
(i) Temperature
(ii) Pressure
(iii) Catalyst.
LEARNING HUB.TZ Page 1
LEARNINGHUBTZ.CO.TZFORM THREE CHEMISTRY MODAL SERIES 12
MODEL EXAMINATION PAPERS
CHEMISTRY FORM THREE
MODEL PAPER 1
NAME…………………………………..CLASS…………………………….……………………TIME 3HOURS
INSTRUCTIONS:-
- This paper consists of three sections- A, AND B
- Answer all questions in section A and B.
- This paper consists of section A, B, and C
- Answer all questions
- You may use the following constants: Na = 23, C = 12, O = 16, IGMV = 22.4dm3 IF = 96500C, NA = 6.02 X 1023
SECTION A.
- Write the letter of the best answer in the box provided below
(i) How would you protect yourself from direct contact with corrosive chemical in the lab?
- Use protective clothing
- Avoid smoking
- Never pour or threw into sinks
- Don’t smell the chemical
(ii) What is the use of a funnel?
- To filter solutions
- To lead fluids into flasks
- To drop liquids onto other chemicals
- Multipurpose
(iii) Which part of a blue Bunsen flame is not so hot?
- The pale blue zone
- The green zone
- The tip
- The base
(iv) Which set of compounds have names ending in ide?
- Acids
- Bases
- Binary
- Inorganic
(v) Which of the following is not a homogenous mixture?
- Alloy
- Brine
- Sugar solution
- Muddy water
(vi) Which method is used to separate oxygen from air?
- Distillation
- Fractional distillation
- Filtration
- Evaporation
(vii) What is term used for a substance resulting from condensation process?
- Distillate
- Sublimate
- Liquid
- Condensate
(viii) What is the colour of phenolphthalein in alkali solution?
- Yellow
- Colourless
- Blue
- Pink
(ix) Which substance will change wet litmus paper from blue to red?
- Lemon juice
- Ash in water
- Sodium carbonate
- Sodium chloride
(x) Which substance produce a pH of 14 when tested
- Alkalis
- Acids
- Metals
- Non-metals.
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
SECTION B.
- Match the lettered items to the numbered items
Numbered item:
(i) Normal salt
(ii) Crystallization by boiling
(iii) Ionic reactions
(iv) Absorbs moisture to form a solution
(v) A more soluble salt
(vi) Softened by boiling
(vii) Nonsense combination of ions for a formula
(viii) Molecular formula
(ix) Most reactive elements in the periodic table
(x) Another name for inert gases
Lettered items
- Deliquescent
- Hydroscopic
- Preparation of soluble salts
- Preparation of insoluble salts
- A compound containing –ve and +ve ions in solution other than OH or H+ ions
- A compound whose hydrogens are party displaced
- Produce precipitates or gases
- Produce ions
- Permanent hardness of water
- Temporary hardness of water
- Atoms are in a simple ratio of combined masses
- Compound has actual number of atoms combined
- Sodium nitrate
- Sodium chloride
- KNa0
- AlCl3
- Noble gases
- Lithium, potassium
- Fluorine, francium
- Air
- Does not have replaceable hydrogen
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
- (a) Draw a well labeled diagram of Downs cell and show the half reactions in the extraction of sodium
(b) What is the effect of temperatures above 800°C in process (a) above?
(c) How can the temperature of 800°C be lowered to 600°C?
- Write ionic equations of the following chemical reactions
(a) Aqueous sodium carbonate reacts with dilute hydrochloric acid
(b) Silver nitrate solution reacts with dilute magnesium chloride solution
(c) Zinc metal is put in a solution of copper sulphate
(d) Barium chloride solution reacts with sodium sulphate solution
(e) Calcium metal is reacted with cold water
- (a) What is molecular formula?
(b) Compound Q was found to have the molar mass of 106 with the following percentage composition by mass 43.4% sodium, 11.3% carbon, 45.5% oxygen. Find the molecular formula of the compound Q and write its chemical name.
- Find solutions A – E have the following pH values: A. ph1 B. PH6 C. PH7 D. PH9 E. PH12
(a) Which of the solutions:
(i) Is exactly neutral
(ii) Is strong acidic
(iii) Is weakly alkaline
(b) Could be a solution of carbon dioxide in water
(c) Will give universal indicator colour (i) Green (ii) Red
(d) Will contain a large concentration of OH ions
(e) Could be a solution of ammonia in water?
- (a) Write the IUPAC names of the following compounds
(i) NaClO3
(ii) K1O3
(iii) NaH
(iv) Ca(NO3)
(v) NO
(b) Write the chemical formula of the following compounds
(i) Lead (II) chloride
(ii) Aluminium hydride
(iii) Sodium hydrogen phosphate (v)
(iv) Sulphur oxide (VI)
(v) Ammonium chloride
SECTION C
- (a) State the chaterliers principle
(b) With the help of an energy level diagrm on the reaction
![]() CaCO3 CaO(g) + CO2(g)
CaCO3 CaO(g) + CO2(g)
?H = + 175.5 kJ. Mol -1
(i) Show weather the reaction is exothermic or endothermic
(ii) Write down the type of the reaction shown by the above equation
(c) What will be the effect on the proportion of calcium carbonate in the equilibrium mixture in the above equation if
(i) The temperature is increased?
(ii) The pressure is increased?
- (a) Define electrolysis
(b) An electric current was passed in series through solutions of calcium chloride and copper (II) sulphate using carbon electrodes in the electrolytes. If 2.5dm3 of chlorine gas was collect at stp what volume of oxygen and what mass of copper were formed at the same time?
- Ammonia in industry is manufactured by Haber process
N2(g) + 3H2(g) 2NH3(g) + 46.2 kJ. mol-1
By the help of a well labeled diagram explain how the above process takes place under the influence of
(i) Temperature
(ii) Pressure
(iii) Catalyst.
LEARNING HUB.TZ Page 1
LEARNINGHUBTZ.CO.TZFORM THREE CHEMISTRY MODAL SERIES 1
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256






