THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM ONE ANNUAL EXAMINATION
CHEMISTRY
TIME: 3 HOURS NOVEMBER 2024
Instructions
1. This paper consists of sections A, B and C with a total of ten (10) questions.
2. Answer all questions. All answers must be written in the spaces provided.
3. Section A and C carry fifteen (15) marks each, Section B carries seventy (70)
marks.
4. All writing must be in blue or black ink except drawings which must be in pencil.
5. All communication devices, calculators and any unauthorized materials are not
allowed in the Mock Assessment room.
6. Write your Mock Assessment Number at the top right corner of every page.
7. The following atomic masses may be used: H = 1, C = 12, O = 16, Na = 23,
S = 32, Ca = 40.
SECTION A (15MARKS)
Answer all questions in this section.
1. For each of the following (i)-(x) choose correct answer from alternative given
i. One of the following apparatus is used to measure a fixed volume of liquids
A. Pipette
B. Burette
C. Measuring cylinder
D. Beaker
ii. Coloured substance can be separated through process called
A. Filtration
B. Chromatography
C. Distillation
D. Sublimation
iii. Which of the groups of substance represents flammable liquid
A. Petrol, Pesticides, Hydrogen
B. Petrol, Sulphuric acid, Methylated spirit
C. Methylated spirit, Petrol, Kerosine
D. Kerosine, Diesel, Hot water
iv. Acid changes colour litmus paper from
A. Blue to Yellow
B. Red to Blue
C. Red to pink
D. Blue to Red
v. Chemistry is a scientific activity because
A. Chemistry is studied in school
B. Chemistry knowledge is acquired through observation experimentation and logical reasoning
C. It is an interesting subject
D. It involves the study of non-living things
vi. On her first experiment, Lilian Dissolved sulphuric acid in water and heat was evolved. In her second experiment she dissolved sugar in water and no heat was evolved or absorbed. She conclude that;
A. Her first experiment was physical change
B. Both Of her experiment were physical change
C. Her second experiment was a chemical change
D. Her first experiment was a chemical change
vii. Loose or floppy clothing is not allowing in the laboratory, why
A. Movement has to be fast
B. It will get wet when water splashes
C. It may catch fire or cause one to fall
D. It cause poor ventilation in the body
viii. Which of the following is not a component of First Aid Kit
A. Googles
B. Gloves
C. Dropper
D. Pair of scissors
ix. If you want Bunsen Burner Flame to have the same colour on the candle flame you must
A. Have air hole completely open
B. Turn on a large supply of gas
C. Have the air hole completely closed
D. Have more candle burning
x. A rapid chemical reaction that release energy in form of light and noticeable heat is called
A. Ignition
B. Reactant
C. Combustion
D. Heating
2. Match the mixture in List A with the corresponding method of separation in List B by writing the letter of correct answer below item number.
| LIST A | LIST B |
| i. Ethanol from water ii. Salt from sea water iii. Rice from husk iv. Oil in sun flower v. Erythrocycles from blood | A. Chromatography B. Filtration C. Solvent extraction D. Fractional Distillation E. Decantation F. Layer separation G. Centrifugation H. Winnowing I. Simple distillation J. Evaporatio |
SECTION B
Answer all question
3. a) Is air a mixture or a compound. Give four reasons
b) A form two student dipped a clean in rod into a cold distilled water in a test-tube and left it for 2 days
i. State what will happen to iron after two days.
ii. Explain the observation if the rod is replaced by a painted nail in the same test tube and left there for 2 days
iii. Explain the observation if cold distilled water will be replaced by a mixture of hot water an oil
c) Explain any two methods that can be used to prevent iron from rusting by giving vivid example
4. a) Differentiate
i. Homogeneous mixture from heterogeneous mixture
ii. Miscible from immiscible liquids
b) Saypalm was in kitchen frying eggs container A and by the same time he submitted solid Iodine in other container which was labelled B
i. What changes were encounted by two substance mention two
ii. State the difference which exists between changes in (a) (i) by providing three points
5. a) Identify types of change involved in each of the following
i. Respiration
ii. Sublimation
iii. Combustion
iv. Distillation
b) Write the chemical symbol of the following
i. Potassium
ii. Sodium
iii. Mercury
iv. Gold
c) Write the most suitable method of separating the following mixture
i. Air
ii. Kerosene and water
iii. Iodine and sand
iv. Syrup
6. a) What do you understand by the term fire
b) Mushi Burned a piece of wood and result were energy and light
i. Name chemical reaction involved
ii. What is the name of given to piece of wood in this reaction
iii. With one example write three areas where chemical reaction in (b) (i) above is applied
7. a) Laboratory technician planned to label warning signs on container found in laboratory. Suggest the name of warning sign he could label on the container with the following;
i. Rat poison
ii. Methylated spirit
iii. Hydrogen peroxide
iv. Concentrated sulphuric acid
v. Cooking gas
b) Briefly explain how you can help an individual who has fainted
8. a) Define a flame
b) Identify factors (2) to consider when choosing a flame
c) Write five properties of luminous flame
9. a) Justine is not interested in studying chemistry. Briefly explain to him five reasons why he should study chemistry.
b) With examples indicate five areas where chemistry is applied.
SECTION C 15 marks
10. a) Differentiate between
i. An element and an atom
ii. A compound and mixture
b) What should you do immediately if;
i. A piece of paper of broken beaker cuts your figure
ii. Chemical splash on your face
iii. Your shirt has caught fire
iv. Your fellow student swallow unknown chemical substance thinking that it was water
c) State the uses of the following in first Aid
i. The pair of scissors
ii. Petroleum jelly
iii. Whistle
iv. Sterile gauze
LEARNINGHUBTZ.CO.TZFORM ONE CHEMISTRY MODAL SERIES 20
MODEL EXAM PAPER 2
CHEMISTRY EXAM
FORM 1
NAME ..CLASS TIME: 2HRS
INSTRUCTIONS:-
- This paper consists of three sections- A, AND B
- Answer all questions in section A and B.
SECTION A: 25 MARKS
- Choose the most correct answer from among of the given alternatives and write its letter in the space provided;
- The following skills acquired through studying chemistry except;
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Chemistry is a scientific activity because;
- Chemistry is studied in schools
- Chemistry is interesting subject studied through data collection and analysis.
- Knowledge of chemistry is acquired through observation, experimentation and logical reasoning.
- It involves study of living and non living things.
- The following are laboratory rules except;
- Turn off water and gas tap after use
- Test any unlabelled substance before using it in the laboratory.
- Put off flames which are not in use
- Remove obstacles from gang ways.
- Splints are among of the first aid kit equipments which of the following sentences set describes its use;
- Used to pick up and holding things
- Used for cutting bandages or pieces of clothes
- Used to support broken bones and is tied using bandages.
- Used for holding pieces of bandages or cloth together.
- The Bunsen burner is likely to contain soot when;
- The air hole is closed
- The air hole is open but not fully
- The air holes of Bunsen burner are completely open.
- The burner is not raised
- The gas supply is high
- Which of the following takes place in experimental stage?
- Rejection of hypothesis
- Acceptance of hypothesis
- Concluding the scientific findings
- Carry out investigations
- In scientific procedure, what would be the next step to follow after experiments?
- Data interpretation
- Conclusion
- Observation
- Investigation
- In her first experiment, Mwajuma dissolved sulphuric acid in water and heat was evolved. In her second experiment he dissolved sugar in water and no heat was evolved or absorbed she conducted that;
- Her first experiment was physical change
- Both of her experiments were physical change
- Her second experiment was chemical change
- Her first experiment was chemical change
- In most general sense the word combustion means .
- Burning substance in oxygen or air.
- Burning compound of carbon in air.
- Burning any fuel in air or oxygen
- By chemical combination accompanied by light and heat in which or more reactants is gaseous
- 10 grams of copper powder were placed initiation tube containing air. The tube was very strongly for half an hour. At the end experiment;
- Copper increased in mass in changed colour
- Copper melted into liquid copper form.
- The total mass of tube and its content decreased.
- Water vapour appeared in the tube.
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
- Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
- Write T for correct statement and F for incorrect statement;
- All materials used for preventing rusting aim at preventing water and oxygen from reaching the iron.
- Accidents always happen in the laboratory.
- All experiments must be done in the fume cupboards.
- Rusting cannot cause corrosive to some articles made up iron or steel.
- Desiccator is an apparatus used to dry solid chemicals.
| i | ii | iii | iv | v |
|
|
|
|
|
|
SECTION B: 75 MARKS
- (a) Define the following terms;
- Combustion .
- Combustible material .
(b) Label the parts marked A, B, C, D, E and F in the diagram below;
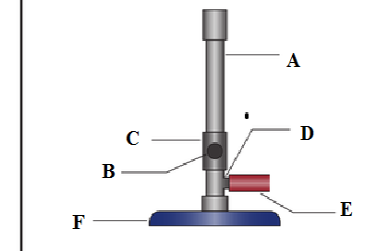
- ..
- ..
- .
- ..
- ..
- ..
(c) What is the importance of parts labelled?
B
C
D .
- Fill the suitable word in the space provided;
(a) Bronze is an alloy formed by mixing and .
(b) Steel is an alloy formed by mixing and .
(c) is a solution in which like solvent used is not water.
(d) .. is used to stir and mix substance uniformly.
- (a) Define the following terms and give one example in each case;
- A chemical symbol
- An element
- A compound
- Suspension
(b) Write names of elements represented by the following symbols
i. Au
ii. Ag .
iii. Hg ..
iv. P ..
v. Sn
- Draw apparatus which are used to perform the following process in the laboratory;
| Process | Apparatus diagram |
| (a) It is used to measure accurate 20/25cm3 of liquid.
|
|
| (b) A flat apparatus at the base used to hold volume of liquid during experiment.
|
|
| (c) Wire placed on top of tripod stand.
|
|
| (d) Used for scooping small quantities of powder or crystalline chemicals.
|
|
| (e) Used for holding heating and mixing liquid it measures estimate volume of liquids.
|
|
- (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
(b) Distinguish between the following;
i. Hypothesis against fact
ii. Deposition against sublimation
(c) Point out any 6 differences between metals and non metals flame;
| Metals | Non metals |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- (a) Briefly explain any five methods of preventing rusting.
(b) A certain pink coloured compound is heated to form blue colour. When water is
added to sample , it changed back to pink.
i. What change is demonstrated by the compound ....
ii. Explain the reasons for your choice.
... .
(c) State the type of changes demonstrated by the following phenomenon;
- Burning of firewood .
- Rotting of meat .
- Heating frying pan ..
- Melting of butterfat
- Change of cloud to rain ..
- Heating of iron rod ..
- Your provided with the diagrams below, study it carefully and answer questions that follows;
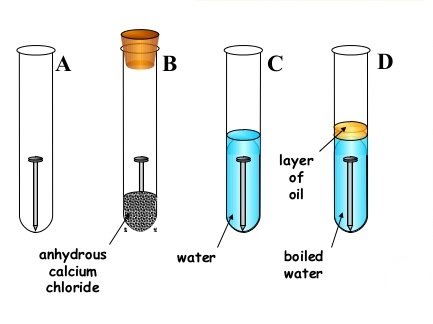
(a) State short and clear description about what will be observed in each test tube A D after 2 or 3 days;
- Test tube A. .
- Test tube B ..
- Test tube C. ..
- Test tube D. ..
(b) Why the water in the test tube D boiled the covered with oil.
(c) What is the function of calcium chloride in test tube B? .
(d) What conclusion can you draw from your experiment?
(e) (i) Give two similarities between combustion and rusting
(ii) Give two differences between combustion and rusting
1 | Page
LEARNINGHUBTZ.CO.TZFORM ONE CHEMISTRY MODAL SERIES 12
MODEL EXAM PAPER 2
CHEMISTRY EXAM
FORM 1
NAME ..CLASS TIME: 2HRS
INSTRUCTIONS:-
- This paper consists of three sections- A, AND B
- Answer all questions in section A and B.
SECTION A: 25 MARKS
- Choose the most correct answer from among of the given alternatives and write its letter in the space provided;
- The following skills acquired through studying chemistry except;
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Chemistry is a scientific activity because;
- Chemistry is studied in schools
- Chemistry is interesting subject studied through data collection and analysis.
- Knowledge of chemistry is acquired through observation, experimentation and logical reasoning.
- It involves study of living and non living things.
- The following are laboratory rules except;
- Turn off water and gas tap after use
- Test any unlabelled substance before using it in the laboratory.
- Put off flames which are not in use
- Remove obstacles from gang ways.
- Splints are among of the first aid kit equipments which of the following sentences set describes its use;
- Used to pick up and holding things
- Used for cutting bandages or pieces of clothes
- Used to support broken bones and is tied using bandages.
- Used for holding pieces of bandages or cloth together.
- The Bunsen burner is likely to contain soot when;
- The air hole is closed
- The air hole is open but not fully
- The air holes of Bunsen burner are completely open.
- The burner is not raised
- The gas supply is high
- Which of the following takes place in experimental stage?
- Rejection of hypothesis
- Acceptance of hypothesis
- Concluding the scientific findings
- Carry out investigations
- In scientific procedure, what would be the next step to follow after experiments?
- Data interpretation
- Conclusion
- Observation
- Investigation
- In her first experiment, Mwajuma dissolved sulphuric acid in water and heat was evolved. In her second experiment he dissolved sugar in water and no heat was evolved or absorbed she conducted that;
- Her first experiment was physical change
- Both of her experiments were physical change
- Her second experiment was chemical change
- Her first experiment was chemical change
- In most general sense the word combustion means .
- Burning substance in oxygen or air.
- Burning compound of carbon in air.
- Burning any fuel in air or oxygen
- By chemical combination accompanied by light and heat in which or more reactants is gaseous
- 10 grams of copper powder were placed initiation tube containing air. The tube was very strongly for half an hour. At the end experiment;
- Copper increased in mass in changed colour
- Copper melted into liquid copper form.
- The total mass of tube and its content decreased.
- Water vapour appeared in the tube.
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
- Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
- Write T for correct statement and F for incorrect statement;
- All materials used for preventing rusting aim at preventing water and oxygen from reaching the iron.
- Accidents always happen in the laboratory.
- All experiments must be done in the fume cupboards.
- Rusting cannot cause corrosive to some articles made up iron or steel.
- Desiccator is an apparatus used to dry solid chemicals.
| i | ii | iii | iv | v |
|
|
|
|
|
|
SECTION B: 75 MARKS
- (a) Define the following terms;
- Combustion .
- Combustible material .
(b) Label the parts marked A, B, C, D, E and F in the diagram below;

- ..
- ..
- .
- ..
- ..
- ..
(c) What is the importance of parts labelled?
B
C
D .
- Fill the suitable word in the space provided;
(a) Bronze is an alloy formed by mixing and .
(b) Steel is an alloy formed by mixing and .
(c) is a solution in which like solvent used is not water.
(d) .. is used to stir and mix substance uniformly.
- (a) Define the following terms and give one example in each case;
- A chemical symbol
- An element
- A compound
- Suspension
(b) Write names of elements represented by the following symbols
i. Au
ii. Ag .
iii. Hg ..
iv. P ..
v. Sn
- Draw apparatus which are used to perform the following process in the laboratory;
| Process | Apparatus diagram |
| (a) It is used to measure accurate 20/25cm3 of liquid.
|
|
| (b) A flat apparatus at the base used to hold volume of liquid during experiment.
|
|
| (c) Wire placed on top of tripod stand.
|
|
| (d) Used for scooping small quantities of powder or crystalline chemicals.
|
|
| (e) Used for holding heating and mixing liquid it measures estimate volume of liquids.
|
|
- (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
(b) Distinguish between the following;
i. Hypothesis against fact
ii. Deposition against sublimation
(c) Point out any 6 differences between metals and non metals flame;
| Metals | Non metals |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- (a) Briefly explain any five methods of preventing rusting.
(b) A certain pink coloured compound is heated to form blue colour. When water is
added to sample , it changed back to pink.
i. What change is demonstrated by the compound ....
ii. Explain the reasons for your choice.
... .
(c) State the type of changes demonstrated by the following phenomenon;
- Burning of firewood .
- Rotting of meat .
- Heating frying pan ..
- Melting of butterfat
- Change of cloud to rain ..
- Heating of iron rod ..
- Your provided with the diagrams below, study it carefully and answer questions that follows;

(a) State short and clear description about what will be observed in each test tube A D after 2 or 3 days;
- Test tube A. .
- Test tube B ..
- Test tube C. ..
- Test tube D. ..
(b) Why the water in the test tube D boiled the covered with oil.
(c) What is the function of calcium chloride in test tube B? .
(d) What conclusion can you draw from your experiment?
(e) (i) Give two similarities between combustion and rusting
(ii) Give two differences between combustion and rusting
1 | Page
LEARNINGHUBTZ.CO.TZFORM ONE CHEMISTRY MODAL SERIES 11
MODEL EXAM PAPER 3
CHEMISTRY EXAM
FORM 1
NAME………………………………………..CLASS……………………………TIME: 2HRS
INSTRUCTIONS:-
- This paper consists of three sections- A, AND B
- Answer all questions in section A and B.
SECTION A. (20 marks)
- Choose the letter of the best answer in the multiple chooses i-x and write the answer in the space provided
(i) What is chemistry? It is the study of
- Chemicals and equipments
- Behavior of matter
- Conservation of matter
- Chemical composition of matter
(ii) A simple proof that some chemical reactions take place in our bodies is that
- We occasionally fall sick
- Doctors tell us so in the hospital
- We eat balanced diet
- The food we eat or the drinks, we take or quite different from the waste products from our bodies
(iii) What is the characteristics of an oxidizing (strong heating) flame
- Yellow
- Blue
- Irregular
- Quiet
(iv) If a chemical substance can easily destroy surface of other substances which warning sign is appropriate for it?
- Corrosive symbol
- Toxic symbol
- Carcinogenic symbol
- Harmful symbol
(v) The following are found in the first aid kit except
- Assorted bondages
- Amoxyl tablets
- Pain killer
- Antiseptic
(vi) Which step in the scientific procedure can be related to an aim of an experiment?
- Statement of a problem
- Formation of hypothesis
- Observation and data collection
- Data analysis and interpretation
(vii) The burning of charcoal, rusting of iron and explosion of hydrogen in air may be classified as
- Dangerous and delicate reaction
- Chemical changes
- Physical changes
- Easily reversible reaction
(viii) Matter exist in three stages these are
- Solid, vapour and gas
- Heat, light and sound
- Liquid, solid and water
- Liquid, solid and gas
(ix) Pick the odd man out from the following
- Destructive distillation of coal
- Fractional distillation of crude oil
- Alcohol production by distillation
- Condensation of steam to get water
(x) An example of a physical change is when
- Firewood burns to form ashes
- Iron nail from rust
- Hydrogen peroxide decomposes to form water and oxygen
- Steam change to clouds and then to rain
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
- Choose a respond from column B which best matches with a statement in column A and write in the space provided
Column A.
(i) Heterogeneous mixture
(ii) Substance which catches fire easily
(iii) Liquid metal
(iv) Used to prevent direct heat to reach the apparatus
(v) Element
(vi) Example of a compound
(vii) Used to date
(viii) Emulsion
(ix) Evaporation
(x) Symbol of zinc
Column B
- Is the process where liquid changes into gaseous
- Is the phenomenon where liquid change into chemical
- Are mixtures which do not mix accordingly
- Sediments deposit at the base
- Carbon fourteen
- Carbondioxide
- Sugar, ethanol and water
- Salt, ammonium chloride and air
- Is the substance which cannot be split up into any simpler substance by any chemical means
- Is the process of splitting up the micro-substance into macro-substance
- Wire gauze
- Tripod stand
- Mercury
- Sodium
- Flammable
- Oxidizing agent
- Oil and water
- Sugar solution
- Zc
- Zn
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
SECTION B. (54 MARKS)
- (a) Metallic shinny lustre is physical property of _________________________ while very weak inter- molecular forced of attraction is the physical property of ______________. High molecular compaction is another characteristics of _____________________________
(b) There is an increase in mass of a substance which burnsin air and this is a characteristics of ___________________________ change. Energy conservation when a substance changes from one state to another is a property of ___________________________ change
- (a) Motion of particles is Radom in _______________________________ and ___________________ state of matter while it is vibratory in ________________________________ state of matter.
(b) if a moving particle takes up ______________________ energy it increases its _____________________ energy and moves __________________________. This may lead to change of _________________________________ for non gaseous substance
- Study the diagram below:



![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() (i) (ii) (iii)
(i) (ii) (iii)
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
(a) Name the three states of matter by the above diagrams
(i) _____________________________________________________
(ii) _____________________________________________________
(iii) _____________________________________________________
(b) Write (i) or (ii) or (iii) against the following statement
A diagram whose particles are closest due to strong attractive force___________________
A diagram whose particles are very far apart due to weakest force of attraction ______________________________
A diagram whose particles are highly compressible __________________
- (a) Draw well labeled diagram of the following laboratory apparatus
| Conical flask | Condenser | Asbestos wire gauze |
|
|
|
|
(b) Write against each name of a laboratory apparatus the function of each of them:
(i) Test tube rack ________________________________________________
(ii) Thermometer ________________________________________________
(iii) Spatula _______________________________________________________
- Draw and name the chemical symbols which will be found with the following chemicals
| Methylated spirit | Sodium cyanide | Sulphuric acid concentrated |
|
|
|
|
- Write “T” for true statement and “F” for force statement
- All heterogeneous mixtures are suspensions __________________
- All suspensions are heterogeneous mixtures ________________
- Smoke in air is a suspension _______________
- One example of emulsion is colour paint _____________
- An example of an alloy is brass _____________
- Air is a compound _____________
- Write English names of the following symbols:
(i) Br __________________________________
(ii) Hg __________________________________
(iii) H ____________________________________
(iv) Na ______________________________________
(v) K __________________________________________
(vi) I ______________________________________________
- (a) Mention three examples of physical change of matter
(i) _______________________________________
(ii) _______________________________________
(iii) _______________________________________
(b) Mention three examples of chemical change of matter
(i) ____________________________________________________
(ii) _____________________________________________________
(iii) _____________________________________________________
- (a) Define the following terms:
(i) First Aid kit __________________________________________________________________________________________________________________________________________________________________________________
(ii) First Aid __________________________________________________________________________________________________________________________________________________________________________________
(b) Write the uses of the following materials of the first aid kit
(i) A pair of scissors ______________________________________________
(ii) Medicated soap _______________________________________________
(iii) Antibiotic solution _________________________________________
(iv) Razor blade ___________________________________________________
SECTION C. (20 MARKS)
- (a) What is a flame?
(b) Write five differences between a luminous flame and a non luminous flame
(c) Draw a well labeled diagram of a luminous flame
(d) Draw a well labeled diagram of a non luminous flame
(e) Mention two advantages of a Bunsen burner over other sources of heat in the laboratory
LEARNINGHUBTZ.CO.TZFORM ONE CHEMISTRY MODAL SERIES 10
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256