FORM ONE CHEMISTRY EXAM SERIES 230
FORM ONE CHEMISTRY EXAM SERIES 230


FORM ONE CHEMISTRY EXAM SERIES 219
FORM ONE CHEMISTRY EXAM SERIES 219
 .
. 

FORM ONE CHEMISTRY EXAM SERIES 208
FORM ONE CHEMISTRY EXAM SERIES 208
OFFICE OF THE PRESIDENT, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY FORM ONE-MID TERM-EXAMINATION
COMPETENCE BASED SECONDARY EXAMINATION SERIES
MARCH-APRIL-2025
TIME: 2HRS 30 MIN
INSTRUCTIONS.
1. This paper consist of section A, B, and C with total of 10 questions.
2. Answer all questions
3. Section A carry fifteen 15 marks, section B seventy 70 marks and fifteen 15 marks in section C.
4. All answers must be written in the spaces provided.
5. Write your Assessment number / Name at the top right hand corner of every page
SECTION A:15 MARKS
1. For each of the items (i)-(x), choose the correct answers from given alternative
i. Which of the following safety equipment is used to wash chemicals off the eyes in case of an accident?
A. Fire extinguisher
B. Eyewash station
C. Fume hood
D. Gas supply system
ii. What is the primary function of a fume hood in a Chemistry laboratory?
A. To store laboratory equipment
B. To provide heat for experiments
C. To safely ventilate harmful gases and fumes
D. To mix and measure chemicals
iii. Why is it important for laboratory doors to open outward?
A. To make the lab look modern
B. To allow easy exit during emergencies
C. To increase the working space inside
D. To prevent unauthorized access
iv. A student wants to measure exactly 25.0 cm³ of a liquid reagent for an experiment. Which apparatus should they use?
A. Beaker
B. Measuring cylinder
C. Burette
D. Pipette
v. What should be done when a strong acid spills on the laboratory bench?
A. Leave it to evaporate
B. Neutralize it with a base like sodium bicarbonate
C. Wash it off with plenty of water
D. Mix it with another acid
vi. What is the best way to safely dispose of chemical waste in the Chemistry laboratory?
A. Pour it down the sink
B. Store it in a labeled chemical waste container
C. Mix it with water and throw it away
D. Burn it in the fume hood
vii. Why is it necessary to wear safety goggles while conducting experiments?
A. To look like a professional scientist
B. To protect the eyes from harmful chemical splashes
C. To avoid smelling strong fumes
D. To read instructions more clearly
viii. Which safety symbol should be found on a container of concentrated sulfuric acid?
A. Flammable
B. Corrosive
C. Toxic
D. Radioactive
ix. A student is heating a substance in a crucible. Which apparatus should they use to safely hold the crucible?
A. Test tube holder
B. Forceps
C. Tripod stand
D. Crucible tongs
x. Which of the following is NOT a correct safety rule for Chemistry laboratory work?
A. Always label all chemical containers
B. Smell chemicals directly to check their identity
C. Turn off the gas supply after use
D. Do not eat or drink in the laboratory
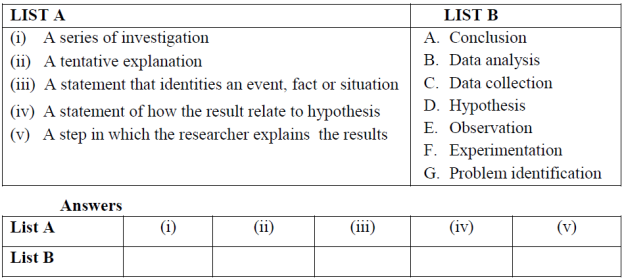
2. Match the chemistry term in LIST B with the correct description given in LIST A.
| LIST A | LIST B |
| (i) The branch of chemistry that deals with carbon and its compounds. (ii) The branch of chemistry concerned with techniques for identifying and analyzing chemical substances. (iii) A chemical process that takes place in the bodies of living things. (iv) The branch of chemistry that studies the structure of matter and mechanisms of reactions. (v) Inorganic substances.
| A. Analytical Chemistry B. Organic Chemistry C. Physical Chemistry D. Biochemistry E. Water
|
SECTION B. 70 MARKS. ANSWER ALL QUESTIONS
3. (a) What are the two key questions that Chemistry tries to answer about substances?
(b) Define the term chemistry and give three branches of chemistry
(c) What makes up substances?
(d) How is chemistry applied in agriculture?
4. (a) Why is it necessary to study chemistry
(b) Mention five home-made items which requires chemistry knowledge
5. (a) How did alchemy contribute to the development of modern Chemistry?
(b) Why did alchemists fail in their effort to make new elements?
6. (a) What is the purpose of a fume hood in a chemistry laboratory?
(b) Why are outward-opening doors important in a chemistry laboratory?
(c) Name three types of flasks commonly used in a chemistry laboratory.
7. (a) What is the purpose of a desiccator?
(b) What is the difference between a luminous and a non-luminous flame?
8. (a) List three safety measures that should be followed in a chemistry laboratory.
(b) What is the function of a pipette?
9. Describe the purpose of a wire gauze in a laboratory setup.
SECTION C: 15 MARKS
10. Explain the relationship between chemistry and other disciplines.
FORM ONE CHEMISTRY EXAM SERIES 193
FORM ONE CHEMISTRY EXAM SERIES 193
THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM ONE ANNUAL EXAMINATION
CHEMISTRY
TIME: 3 HOURS NOVEMBER 2024
Instructions
1. This paper consists of sections A, B and C with a total of ten (10) questions.
2. Answer all questions. All answers must be written in the spaces provided.
3. Section A and C carry fifteen (15) marks each, Section B carries seventy (70)
marks.
4. All writing must be in blue or black ink except drawings which must be in pencil.
5. All communication devices, calculators and any unauthorized materials are not
allowed in the Mock Assessment room.
6. Write your Mock Assessment Number at the top right corner of every page.
7. The following atomic masses may be used: H = 1, C = 12, O = 16, Na = 23,
S = 32, Ca = 40.
SECTION A (15MARKS)
Answer all questions in this section.
1. For each of the following (i)-(x) choose correct answer from alternative given
i. One of the following apparatus is used to measure a fixed volume of liquids
A. Pipette
B. Burette
C. Measuring cylinder
D. Beaker
ii. Coloured substance can be separated through process called
A. Filtration
B. Chromatography
C. Distillation
D. Sublimation
iii. Which of the groups of substance represents flammable liquid
A. Petrol, Pesticides, Hydrogen
B. Petrol, Sulphuric acid, Methylated spirit
C. Methylated spirit, Petrol, Kerosine
D. Kerosine, Diesel, Hot water
iv. Acid changes colour litmus paper from
A. Blue to Yellow
B. Red to Blue
C. Red to pink
D. Blue to Red
v. Chemistry is a scientific activity because
A. Chemistry is studied in school
B. Chemistry knowledge is acquired through observation experimentation and logical reasoning
C. It is an interesting subject
D. It involves the study of non-living things
vi. On her first experiment, Lilian Dissolved sulphuric acid in water and heat was evolved. In her second experiment she dissolved sugar in water and no heat was evolved or absorbed. She conclude that;
A. Her first experiment was physical change
B. Both Of her experiment were physical change
C. Her second experiment was a chemical change
D. Her first experiment was a chemical change
vii. Loose or floppy clothing is not allowing in the laboratory, why
A. Movement has to be fast
B. It will get wet when water splashes
C. It may catch fire or cause one to fall
D. It cause poor ventilation in the body
viii. Which of the following is not a component of First Aid Kit
A. Googles
B. Gloves
C. Dropper
D. Pair of scissors
ix. If you want Bunsen Burner Flame to have the same colour on the candle flame you must
A. Have air hole completely open
B. Turn on a large supply of gas
C. Have the air hole completely closed
D. Have more candle burning
x. A rapid chemical reaction that release energy in form of light and noticeable heat is called
A. Ignition
B. Reactant
C. Combustion
D. Heating
2. Match the mixture in List A with the corresponding method of separation in List B by writing the letter of correct answer below item number.
| LIST A | LIST B |
| i. Ethanol from water ii. Salt from sea water iii. Rice from husk iv. Oil in sun flower v. Erythrocycles from blood | A. Chromatography B. Filtration C. Solvent extraction D. Fractional Distillation E. Decantation F. Layer separation G. Centrifugation H. Winnowing I. Simple distillation J. Evaporatio |
SECTION B
Answer all question
3. a) Is air a mixture or a compound. Give four reasons
b) A form two student dipped a clean in rod into a cold distilled water in a test-tube and left it for 2 days
i. State what will happen to iron after two days.
ii. Explain the observation if the rod is replaced by a painted nail in the same test tube and left there for 2 days
iii. Explain the observation if cold distilled water will be replaced by a mixture of hot water an oil
c) Explain any two methods that can be used to prevent iron from rusting by giving vivid example
4. a) Differentiate
i. Homogeneous mixture from heterogeneous mixture
ii. Miscible from immiscible liquids
b) Saypalm was in kitchen frying eggs container A and by the same time he submitted solid Iodine in other container which was labelled B
i. What changes were encounted by two substance mention two
ii. State the difference which exists between changes in (a) (i) by providing three points
5. a) Identify types of change involved in each of the following
i. Respiration
ii. Sublimation
iii. Combustion
iv. Distillation
b) Write the chemical symbol of the following
i. Potassium
ii. Sodium
iii. Mercury
iv. Gold
c) Write the most suitable method of separating the following mixture
i. Air
ii. Kerosene and water
iii. Iodine and sand
iv. Syrup
6. a) What do you understand by the term fire
b) Mushi Burned a piece of wood and result were energy and light
i. Name chemical reaction involved
ii. What is the name of given to piece of wood in this reaction
iii. With one example write three areas where chemical reaction in (b) (i) above is applied
7. a) Laboratory technician planned to label warning signs on container found in laboratory. Suggest the name of warning sign he could label on the container with the following;
i. Rat poison
ii. Methylated spirit
iii. Hydrogen peroxide
iv. Concentrated sulphuric acid
v. Cooking gas
b) Briefly explain how you can help an individual who has fainted
8. a) Define a flame
b) Identify factors (2) to consider when choosing a flame
c) Write five properties of luminous flame
9. a) Justine is not interested in studying chemistry. Briefly explain to him five reasons why he should study chemistry.
b) With examples indicate five areas where chemistry is applied.
SECTION C 15 marks
10. a) Differentiate between
i. An element and an atom
ii. A compound and mixture
b) What should you do immediately if;
i. A piece of paper of broken beaker cuts your figure
ii. Chemical splash on your face
iii. Your shirt has caught fire
iv. Your fellow student swallow unknown chemical substance thinking that it was water
c) State the uses of the following in first Aid
i. The pair of scissors
ii. Petroleum jelly
iii. Whistle
iv. Sterile gauze
FORM ONE CHEMISTRY EXAM SERIES 187
FORM ONE CHEMISTRY EXAM SERIES 187
PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
MID-TERM EXAMS – AUG/SEPT – 2024
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A
Answer ALL questions.
i. Which is not an importance of studying chemistry;
- Understand how matter undergo chemical change
- Make various medical products
- Understand the use of chemicals in agriculture
- Help us enter into various careers.
ii. A mother at home can apply chemistry to;
- Weed flowers in the garden
- Dusting the floor of her house
- Weighing maize flour
- Cooking food in the kitchen
iii. What real takes place during sacrificial protection of iron from rusting?
- The reactive metal remains uncorroded
- The reactive metal is corroded
- The reactive metal and iron are corroded
- The iron is corroded
iv. Which of the following warning signs is likely to appear on a bottle containing concentrated nitric acid in the laboratory?
- Corrosive
- Explosive
- Irritant
- Flammable
v. What is the best definition of Brownian motion?
- Uniform movement of particles
- A motion discovered by Robert Brownian
- A random movement of particles of gas and liquid
- A random movement of particles of gas and solid
vi. What is usually found in the chimney of a Bunsen burner when it is on use
- Oxygen
- The fuel gas
- A mixture of oxygen and the flammable
- Non of the above
vii. A Bunsen burner flame will produce luminous flame when;
- Gas tap is fully opened
- Air holes of Bunsen burner is fully closed
- Air holes of Bunsen burner is fully opened
- Sufficient gas is supplied to the burner
viii. A metal that is usually in liquid form is called;
- Mercury
- B. Bromine
- C. Iron
- D. Sulphur
ix. A symbol which represents the substances which can catch fire easily is;
- Flammable
- B. Toxic
- C. Explosive
- D. Radioactive
x. When anhydrous copper(II) sulphate is left on the glass turns blue. This confirms presence of ………………………. in air;
- Carbon dioxide
- Dust particles
- Water vapour
- Noble gases
- Write the letter of the best match from column B against a name of the element in column A.
| COLUMN A | COLUMN B |
|
|
SECTION B
- (a) What is the aim of using warning sign on containers in the chemistry laboratory?
(b) Name one example of a protective symbol
- Write name of one chemicals under each of the following
- Oxidant/oxidizing agent
- Toxic/poison
- Harmful/ irritant
4. (a) What is a flame?
(b) Write five differences between a luminous flame and a non luminous flame
(c) Draw a well labeled diagram of a luminous flame
(d) Draw a well labeled diagram of a non luminous flame
(e) Mention two advantages of a Bunsen burner over other sources of heat in the laboratory
5. (a) Give the reason to why the chemicals in the laboratory should be labelled and well closed after use.
(b) The shelves in the laboratory should be labelled and constructed in a way that it is at the eye level and not above the eye level. Give the reason behind the statement.
6. Define the following term;
- Air
- Saturated solution
- Unsaturated solution
- Super – saturated solution
- (a) What is distillation
7. (a)mention three branches of science
b) Who is a chemist?
c) List 5 places where chemistry is applied
d) Name three cleaning agents made by chemistry knowledge
8. a) Define the term class B fire.
b) i) Mention two combustible materials in class B fire
ii) Why is water not used to put off oily fires?
iii) Your friends clothes have caught fire. In order to extinguish the fire, you have decided to cover her with a blanket. What is the function of the damp blanket?
c) i) Why is air a mixture and not a compound?
ii) Why is rusting of iron a chemical change?
9. a) Briefly explain the following,
i) Iron left outside for some time will have a brown colour.
ii) Things made of iron rust faster at the coast than at upcountry areas.
iii) When carbon dioxide is passed through lime water for some times, the limewater turns milky.
SECTION C
10. (a) What are the effect if on to fails to follow the following laboratory instructions;
- Failure to follow instructions guided.
- Improper disposal of broken glass materials ……………………………………………………………………………………………….
(d) Write the functions of the following laboratory tools 2 functions;
Round bottomed flask
i. …………………………………………………………………………………………..
ii. …………………………………………………………………………………………..
Burette
i. ……………………………………………………………………………………….
ii. ……………………………………………………………………………………….
FORM ONE CHEMISTRY EXAM SERIES 178
FORM ONE CHEMISTRY EXAM SERIES 178
THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY FORM ONE TERMINAL EXAMINATION
CODE 032
TIME: 2:30 HOURS
INSTRUCTIONS.
- This paper consists of section A, B and C with the total number of ten(10) questions
- Answer all questions in each section
- Section A carries (15) marks, section B (70) marks and section C carries (15) marks
- All writing must be in blue/black ink except drawing which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A (15 Marks)
Answer all questions in this section
1. For each of the items (i) – (x) choose the correct answer from among the given alternatives and write its letter beside the item number in the answer sheet provided
- A new substance is formed when baking soda and vinegar are mixed. This is evidence of:
A. A physical change
B. A chemical change
C. A temperature change
D. A change in size
- Which product heavily relies on the work of chemists in its creation?
A. Fertilizers for growing crops
B. Fabrics for durable clothing
C. Concrete for building structures
D. All of the above
- To design a water filtration system for a community, an engineer needs knowledge of chemistry to:
A. Determine the best materials for capturing impurities.
B. Understand how different substances interact.
C. Predict the outcomes of different filtering methods.
D. All of the above
- During an experiment, you notice an unexpected color change. To follow good scientific practice, you should:
A. Record the change and try to explain why it occurred
B. Ignore it and continue as planned
C. Stop the experiment immediately
D. Tell your friend but don't write it down.
- Wearing safety goggles in the lab is essential because they:
A. Protect your eyes from potential chemical splashes.
B. Make you look like a scientist.
C. Prevent you from touching your face.
D. Help you see the experiment more clearly.
- You're unsure how to dispose of a chemical after an experiment. What should you do?
A. Pour it down the sink
B. Mix it with another chemical to see what happens.
C. Leave it at your workstation for the teacher.
D. Ask your teacher for specific disposal instructions.
- Your friend accidentally gets a small amount of a chemical on their hand. They should:
A. Quickly go to the chemical wash station.
B. Wipe it off on their clothes.
C. Tell a teacher and wait for directions.
D. Try to ignore the feeling.
- You need to heat a liquid as part of your experiment. Which apparatus is the safest and most appropriate?
A. Beaker and Bunsen burner
B. Graduated cylinder and candle
C. Erlenmeyer flask and hot plate
D. Test tube and lighter
- A triangular sign with a flame symbol means the substance presents a:
A. Electrical hazard
B. Biohazard
C. Fire hazard
D. Environmental hazard
- Seeing a test tube with cracks on a chemical bottle likely indicates:
A. The chemical is very old.
B. The chemical may be heat sensitive.
C. The chemical could be damaged or unsafe.
D. The chemical is perfectly safe to use.
2. Match the laboratory apparatus in Column A to its correct use in Column B.
| Column A | Column B |
| A. Heating liquids or solids B. Holding and mixing liquids C. Storing liquids for long periods of time (with a stopper) D. Measuring precise volumes of liquids E. Performing small-scale reactions
|
SECTION B: 70 MARKS
3. The following are laboratory apparatus used in Chemistry. Name them and give their uses.
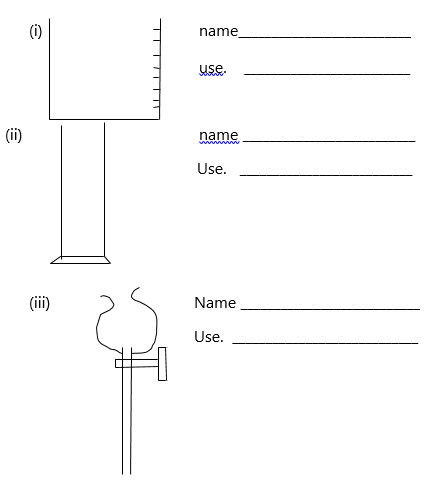
(b). Give two reasons why most laboratory apparatus are made of glass.
4.(a) Name three apparatus that can be used to measure accurate volume
(b) Classify the following as chemical change or physical change;
- Burning of candle
- Burning of paper
- Rusting
- Evaporation of water
- Sublimation of iodine
(c) Name four kinds of materials that can be used to make laboratory apparatus
5. (a) Define the term chemistry
(b) Name areas where knowledge of chemistry finds applications.
(c) Name four home made products which are made using the knowledge of chemistry
6.(a) What is a chemistry laboratory?
(b) Explain why a laboratory is different from other buildings
(c) Mention accidents that can occur in laboratory
7.(a) Define first aid
(b) Why is first Aid important to an accident victim?
© Name four items that can be used when giving first aid to an accident victim
8. (a) Define the term matter
(b) Name three states of matter
(c) Explain why it is possible to separate of mixture of sodium chloride and Iodine
9. (a) Define the following terms
- An atom
- Molecule
- Element
(b) Differentiate between a chemical change and a physical change
SECTION C: 15 Marks
10.(a) Complete the following table;
| Element | Symbol | Element | Symbol |
| Sodium |
|
| Hg |
|
| K | Copper |
|
| Sulphur |
|
| C |
| Iron |
| Hydrogen |
|
(b) Discuss the importance of studying chemistry.
FORM ONE CHEMISTRY EXAM SERIES 171
FORM ONE CHEMISTRY EXAM SERIES 171
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES,
MID TERM ONE – MARCH-2024
CHEMISTRY FORM ONE
TIME 2HOURS
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A(30 MARKS)
- For each of the following items (i-x).Choose the correct answer from given alternatives and writer its letter besides the item number in the box provided
- Which are the common activities done in the chemistry Laboratory
- Exhibition
- Demonstration
- Exercise
- Experiment
- The following substances are constituents of first aid kit in chemistry Laboratory except
- Petroleum gel
- Iodine tincture
- Cotton wool
- Plaster of pans
- What is the suitable method of separating a mixture of sand and Ammonium Chloride?
- Magnetization
- Decantation
- Sublimation
- Simple distillation
- Which one is an example of liquid solution
- Dental Amalgam
- Fresh milk
- Alloys
- vinegar
- Which is the suitable alternative heat source to be used in absence of Bunsen burner?
- Torch and spirit burner
- Torch and kerosene stove
- Kerosene stove and spirit burner
- Firewood and torch
- Which state is involved when drying wet clothes?
- Liquid to solid
- Solid to gas
- Gas to liquid
- Liquid to gas
- Fainting is a suddenly loss of
- Confidence
- Weight of the body
- Water in the body
- Consciousness
- Which statement given a clear meaning of chemistry
- The study of matter in relation to energy
- The study of nature and properties of matter
- Study of matter and arrangement of particles
- The study of matter and chemical reaction
- Which of the following is a metal
- Water
- Chlorine
- Sodium
- Nitrogen
- Matter is anything that has
- Volume and occupying space
- Mass and occupies space
- Mass And occupies density
- Density and space
- Match each item in LIST A with correct response in list B by writing its letter below the item number on table provided
| LIST A | LIST B |
|
|
SECTION B
(70 MARKS)
Answer all questions in this section
- Using diagram explain the arrangement of particles in solid, liquid and gas
- (a) What is Laboratory?
(b) Give four features of a laboratory
(c) Mention three accidents that can occur in laboratory
- (a) What is Chemistry?
(b) Johanes is a form one student who is very reluctant to study chemistry. Advice him on the importance of studying Chemistry.
- (a)what do you understand by first aid
(b) Explain five importance of first aid
(c) Mention three accidents that can occur at home.
- Chemistry is very important for technological advancement of a country.
(i) Mention four areas where knowledge of chemistry is applied
(ii) Name products made by chemistry knowledge in the following fields
- Home items
- Agriculture
- Cosmetics
- Safety in laboratory is very important.
- What do you understand by the term safety
- Mention 8 Laboratory rules that should be observed
- First aid kit is a box containing items to be used during first aid. Give the use of the following items
- Cotton pads
- Pain killer
- Antibiotics
- Soap
- Gloves
- Warning signs are very important in guaranteeing safety of uses in laboratory. Basing your answer on examples of substances describe five warning signs. Use diagrams correctly
FORM ONE CHEMISTRY EXAM SERIES 160
FORM ONE CHEMISTRY EXAM SERIES 160
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
ANNUAL EXAMS – NOVEMBER – 2023
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A. (15 MARKS)
- For each of the item number (i) – (x) Choose the correct answer from given alternatives.
- A simple proof that chemical reaction takes place in our bodies is that;
- We eat balanced diet
- Doctor tells us so in hospital
- We occasionally fall sick
- The food we take is quite different from waste that comes from our bodies
- There is no proof
- What change of state is involved when drying wet clothes?
- Gas to solid
- Liquid to gas
- Gas to liquid
- Solid to gas
- Liquid to solid
- Which is suitable alternative heat source to be used in absence of Bunsen burner?
- Kerosene stove and torch
- Torch and spirit burner
- Kerosene, stone and spirit burner
- Fire wood and torch
- Torch and candle
- Sodium is abbreviated as Na but both letters are exclusive in the word sodium, this is because,
- The Latinized as naterite
- Comes from the world Natural
- The Latin name of sodium is Natritium
- It is derived from two letters of its English name
- It is derived from English name
- Fire triangle is the component required for a fire to start. This includes
- Heat, Fuel, Energy
- Heat, fuel, and air
- Heat, water, and Energy
- Heat, Iron and light
- Heat, light and Energy
- Which of the following is unlikely to produce soot?
- Complete combustion
- Incomplete combustion
- Limited supply of air burning
- Luminous flame
- Air holes open
- Mixture of cooking Oil and water can be termed as
- Suspension
- Solution
- Emulsion
- Saturation
- solvent
- the process of coating iron or steel with zinc is known as
- Zinc painting
- Tin plating
- Alloying
- Electroplating
- Galvanizing
- Which among the following lists contain the items transported that are produced as a result of application of chemistry?
- Dyes, tyres and fuel
- Coolant, fuel and Lubricants
- Pants, lubricants and pesticide
- Lubricant, tyres, and drugs
- Coolant plant Dyes
- Which of the following group of substances represents flammable liquids
- Petrol, pesticide and hydrogen
- Petrol sulphuric acid and methylated spirit
- Methylated spirit, Petrol and Kerosene
- Kerosene, Diesel and hot water.
- Oxygen, Hydrogen peroxide, Petrol
- Match the materials in LIST A with correct method of preventing it from rusting in LIST B by writing the letter of correct answer below item number in table provided.
| LIST A | LIST B |
|
|
SECTION B. (70 Marks)
Answer all questions in this section
- (a)Briefly explain two conditions necessary for a substance to be called matter
(b)Differentiate solution from suspension using four points.
- (a) Give one (1) reason to support the following statements.
- A luminous flame in said to be safer and less likely to cause accident in the laboratory.
- Chemical which are not correctly labelled should not be used in laboratory
- The component of a mixture of water and cooking oil are separated using layer separation method.
(b) Give two examples of apparatus that are made of
- Porcelain
- Plastic material
- Glass material
- Iron materials
- (a) Describe four importance of first Aid to an accident victim.
(b) Mary dipped a clean iron rod into cold distilled water in a test tube and left it four 2days. Explain Observation after two days.
- State what will happen to the iron rod after 2 days
- Outline three things which cause process in (b) above
- How could these student Prevent the process above despite of the conditions state. Give two method
- (a)What do you understand by the world fire?
(b) Josephine burnt a piece of wood and the results were energy and light
- Name the chemical reaction involved
- What is the name given to a piece of wood in this reaction?
- With One example write three areas where chemical reactions in (b) (i) above is applied
- (a)Identify types of change involved in each of the following, weather physical or chemical change
- Respiration
- Sublimation
- Combustion
- Distillation
(b) Write the chemical symbols of the following elements.
- Potassium
- Sodium
- Mercury
- Gold
- (a)Halima needed to measure the volume of water in a chemistry laboratory. Which apparatuses. Should be used (3 apparatuses)
(b)Name four accidents that needs first Aid procedures in Laboratory
(c) With reason state one method suitable to separate each of following
- Ethanol and water
- Cooking and water
- Ammonium chloride and sand
- (a)Most of famers in rural areas were interested to know how the knowledge of chemistry is necessary in Agriculture activities. Assume you are a chemist; educate these farmers on application of chemistry in agriculture by giving five points.
(b)Why is Non-luminous flame better than luminous flame in heating?
SECTION C (15 Marks)
- Scientific procedures are steps used by scientists when finding answer to scientific problems. Write the steps which correspond to each of the following
- Kelvin was not feeling well. She went to see a medical doctor at Mawenzi hospital
- The Doctor asked Kelvin Several questions on how he was felling
- The doctor Ordered Kelvin body temperature, blood and Urine sample for Observation in the laboratory.
- The laboratory technician diagnosed Malaria parasite in Kelvin Blood.
- The Doctor confirmed that Kelvin had Malaria and prescribed medicine for him.
(b)Why is scientific procedure? Give two points
(c) State three areas where scientific procedures are applied
FORM ONE CHEMISTRY EXAM SERIES 148
FORM ONE CHEMISTRY EXAM SERIES 148
PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
MID-TERM EXAMS – AUGUST – 2023
TIME: 2:30 HRS
INSTRUCTIONS
- Thus paper consists of section A, B and C with total of ten (10) questions
- Answer all questions
- All writing must be in blue or Black in except drawing which must be in pencil
- Section A and C carry fifteen (15) marks
- Cellular phones and any unauthorized material are not allowed in assessment room
SECTION A
- For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
- When a chemistry studies a substance, he/she is interested in its
- Force of Attraction
- Shape
- Smell
- properties
- Chemistry is one of the science which deals with,
- alkalinity and Basiaty of substance
- the study of cells
- composition, properties and behavior of matter
- chemical changes
- Which of the following illustrates a chemical reaction taking place in our body
- Falling sick
- Digestion
- Respiration
- Salvation
- Monica found a bottle with chemical which has a source of Oxygen, what warning sign is vicety to be found on it?
- Flammable
- Explosive
- Oxidizing
- Corrosive
- Which best idea can you have about Uranium, Radon, and carbon – 14
- Neither radioactive nor carcinogenic materials
- Radioactive and carcinogenic materials
- Radioactive but not carcinogenic materials
- Non-radioactive carcinogenic material
- Kipps apparatus in used in laboratory for
- Obtaining continuous supply of gas
- Separation of gases
- Drying gases
- Obtaining Pure gases
- A solution is formed when
- A solvent is dissolved in a solute
- A solute is dissolved in a solvent
- A mixture is dissolved in a solvent
- Water dissolved crystals
- Point out the odd mom out in the following groups of elements
- Zinc, sulphur, sodium
- Copper sodium, Iron
- Alluminium, sodium, Zinc
- Sodium, zinc copper
- Separation of the constituents of a mixture by fractional distillation is possible when constituents in the mixture differ in their
- Boiling point
- Solubility
- Melting point
- Freezing point
- The process used to separate a mixture of salt and water is
- Evaporation
- Filtration
- Simple distillation
- sublimation
- Match each item in LIST A with a correct response in LIST B by writing its letter below the number of the corresponding item in table provided.
| LIST A | LIST B |
|
|
SECTION B (70 MARKS)
Answer all questions from this section
- (a)The material resulting from condensation is called condensate. Derive process resulting from following material
- Distillate______
- Filtrate ____
- Sublimate _____
(b)Write the proper method of separating mixtures against each of the following substances
- Oil in seed
- Sand mixed with iodine crystal
- Alcohol mixed with water
- Mixture of salt and milk
- (a) Give best term that fits description below,
- John gave a tentative explanation of why iron rusts when exposed in air
- Mary followed, all steps required in carrying out scientific investigation
- Juma used a region of burning gas to heat in laboratory
(b)What benefits can Salma gain by studying chemistry? Give four
- (a)Write two examples of each of the following
- Heterogeneous mixture
- Homogeneous mixture
- Compound
(b)State the method used to separate the following mixtures and retain their components
- Muddy water
- Common salt
- Oil and water
- Ink
- (a)Supply the suitable word in space provided
- _______ is a mixture of pure metal with another element
- Brass is made by mixing __________ and _______
- Steel in made by mixing ___________ and ___________
- A steel with high content of iron is called _________
- Liquid which mix together completely are called __________
(b) Define the following terms
- Air
- Saturated solution
- Unsaturated solution
- (a)Write chemical symbols of the following chemical names of elements
- Copper
- Sodium
- Zinc
- Aluminum
- Iron
(b)Write chemical names of the following chemical symbols of elements
- S ________
- H ________
- O _______
- N _______
- Cl _______
- (a)Write five differences between luminous and Non-Luminous flame
(b)State advantages of Bunsen burner as sources of heat
- (a)Explain the following
- Laboratory doors should open outwards
- Laboratory should have large windows and doors
- Laboratory should have fume chambers
- Laboratory should have water supply system
(b)State two causes of accident in laboratory
- (a)Define the following terms
(i) First Aid (ii) First Aid Kit
(b) Each student required to learns the basics of first aid for helping accident victims. What are the importances of helping accident victims? Four reasons
(c)Shock is a condition in which the body system is unable to take enough blood to the vital Organs Explain the procedures to follow in giving help for a shock victim
FORM ONE CHEMISTRY EXAM SERIES 138
FORM ONE CHEMISTRY EXAM SERIES 138
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM ONE
TERMINAL EXAMS-MAY– 2023
033
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carry seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
1. For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
(i) Which of the following is the correct sequence of the last two steps you should follow during the scientific procedure?
- Hypothesis formulation and conclusion
- Observation and problem identification
- Experimentation and conclusion
- Problem identification and hypothesis formulation
- Interpretation of data and conclusion.
(ii) Which of the following is not a component of the First Aid Kit?
- Goggles
- A pair of scissors
- Dropper
- Gloves
- Razor blade
(iii) Which one of the following processes is a chemical change?
- Butter melts on warm toast
- Water evaporates from the surface
- Juice in a bottle freezes
- Food scrap turns into compost
- Wet cloth dries
(iv) When you melt a piece of iron, it undergoes:
- Sublimation
- Physical change
- Chemical change
- Combination
- Synthesis
(v) The most abundant element on the earth is:
- carbon
- iron
- nitrogen
- Oxygen
- Sulphur
(vi) Petrol is an example of:
- corrosive substance
- flammable substance
- irritating substance
- toxic substance
- Oxidant
(vii) The boiling point of pure water at sea level is 1000C and that of ethanol is 780C. The mixture of ethanol and water can be separated by:
- Filtration process
- Fractional distillation process
- Layer separation process
- Sublimation process
- Evaporation
(viii) When a chemist studies a substance he/she is interested in its:
- Force of attraction
- Properties
- Shape
- Smell
- Density
(ix) In the Bunsen burner a sooty flame is most likely to be formed when the:
- Air holes are fully closed
- Air holes are opened
- Flame is noisy
- Flame is smaller and hotter
- Methane gas is used
(x) The process by which water is converted into water vapour or steam is called:
- Condensation
- Evaporation
- Precipitation
- Transpiration
- Fusion
2. Match the terms in LIST B with the correct description in LIST A and write the letter of correct answer in spaces provided.
| LIST A | LIST B |
|
|
SECTION B. 70 MARKS
ANSWER ALL QUESTIONS FROM THIS SECTION.
3. (a) Define the following terms:
(i) Brownian motion
(ii) Compound
(b) Identify whether the following is a physical or chemical change:
(i) Cutting aluminium foil into pieces
(ii) Lighting a match.
(c) How can you separate the following mixtures? Briefly explain.
(i)Water and kerosene
(ii)Salt and water
(iii)Ethanol and water
4. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
5. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv. Sterile gauze
6. (a) Differentiate the following terms:
(i) An element and an atom
(ii) A compound and a mixture
(b) Melting of ice and burning of papers are two processes that happen almost daily in our life.
(i) Identify the two actions as being physical or chemical change.
- Ice melting __________________________________________________
- Paper burning ________________________________________________
(ii) Mention two significant things that happen when the paper is burnt:
(c) What are the names of elements represented by the following symbols?
(i) K _________________________________________________________
(ii) S _________________________________________________________
(d) Why some elements have their symbols written in one letter while others have two letters? (e.g. N and Na)
7. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
8. Describe the eight stages of lighting a Bunsen Burner
9.(a) Explain each of the following terms;
- Burn
- Bruises
- Fainting.
(b) Write the differences between metals and Non-Metals
10.
(a) Give the use of each of the following components which are found in the First Aid kit:
(i) Plaster .........
(ii) A pair of scissors
(iii) Cotton wool .........
(iv) Gloves .......
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
FORM ONE CHEMISTRY EXAM SERIES 129
FORM ONE CHEMISTRY EXAM SERIES 129
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM ONE
TERMINAL EXAMS-MAY– 2023
033
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carry seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
1. For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
(i) Which of the following is the correct sequence of the last two steps you should follow during the scientific procedure?
- Hypothesis formulation and conclusion
- Observation and problem identification
- Experimentation and conclusion
- Problem identification and hypothesis formulation
- Interpretation of data and conclusion.
(ii) Which of the following is not a component of the First Aid Kit?
- Goggles
- A pair of scissors
- Dropper
- Gloves
- Razor blade
(iii) Which one of the following processes is a chemical change?
- Butter melts on warm toast
- Water evaporates from the surface
- Juice in a bottle freezes
- Food scrap turns into compost
- Wet cloth dries
(iv) When you melt a piece of iron, it undergoes:
- Sublimation
- Physical change
- Chemical change
- Combination
- Synthesis
(v) The most abundant element on the earth is:
- carbon
- iron
- nitrogen
- Oxygen
- Sulphur
(vi) Petrol is an example of:
- corrosive substance
- flammable substance
- irritating substance
- toxic substance
- Oxidant
(vii) The boiling point of pure water at sea level is 1000C and that of ethanol is 780C. The mixture of ethanol and water can be separated by:
- Filtration process
- Fractional distillation process
- Layer separation process
- Sublimation process
- Evaporation
(viii) When a chemist studies a substance he/she is interested in its:
- Force of attraction
- Properties
- Shape
- Smell
- Density
(ix) In the Bunsen burner a sooty flame is most likely to be formed when the:
- Air holes are fully closed
- Air holes are opened
- Flame is noisy
- Flame is smaller and hotter
- Methane gas is used
(x) The process by which water is converted into water vapour or steam is called:
- Condensation
- Evaporation
- Precipitation
- Transpiration
- Fusion
2. Match the terms in LIST B with the correct description in LIST A and write the letter of correct answer in spaces provided.
| LIST A | LIST B |
|
|
SECTION B. 70 MARKS
ANSWER ALL QUESTIONS FROM THIS SECTION.
3. (a) Define the following terms:
(i) Brownian motion
(ii) Compound
(b) Identify whether the following is a physical or chemical change:
(i) Cutting aluminium foil into pieces
(ii) Lighting a match.
(c) How can you separate the following mixtures? Briefly explain.
(i)Water and kerosene
(ii)Salt and water
(iii)Ethanol and water
4. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
5. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv. Sterile gauze
6. (a) Differentiate the following terms:
(i) An element and an atom
(ii) A compound and a mixture
(b) Melting of ice and burning of papers are two processes that happen almost daily in our life.
(i) Identify the two actions as being physical or chemical change.
- Ice melting __________________________________________________
- Paper burning ________________________________________________
(ii) Mention two significant things that happen when the paper is burnt:
(c) What are the names of elements represented by the following symbols?
(i) K _________________________________________________________
(ii) S _________________________________________________________
(d) Why some elements have their symbols written in one letter while others have two letters? (e.g. N and Na)
7. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
8. Describe the eight stages of lighting a Bunsen Burner
9.(a) Explain each of the following terms;
- Burn
- Bruises
- Fainting.
(b) Write the differences between metals and Non-Metals
10.
(a) Give the use of each of the following components which are found in the First Aid kit:
(i) Plaster .........
(ii) A pair of scissors
(iii) Cotton wool .........
(iv) Gloves .......
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
FORM ONE CHEMISTRY EXAM SERIES 128
FORM ONE CHEMISTRY EXAM SERIES 128
PRESIDENT OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSESSMENT
033 CHEMISTRY FORM ONE
MID-TERM EXAMS-MARCH – 2023
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carries seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
1. For each of the items (i) – (x), choose the correct answer from among the given alternatives and write its letter in the box provided.
- Chemistry is one of the science which deals with;
- Alkalinity and basicity of substance
- The study of body cells
- Composition, propertier and behavior of matter
- Chemical changes
- In chemistry experiments test
- Data
- Problem
- Hypothesis
- Observation
- When a chemists studies a substance, he/she is interested in its
- Force of attraction
- Shape
- Small
- Properties
- Chemistry is defined as
- The scientific study of matter, compound and chemical reaction
- The scientific study of compounds, mixture and organic substances
- The scientific study of composition structure and properties of matter.
- The study of relation between
- A chemist should acquire all of the following skills except.
- Experimentation
- Observation
- Problem identification
- Surgery
- Which of the following sets of apparatus contains exact volume measuring items?
- Pipette, burette, thermometer
- Pipette burette, measuring cylinder
- Flasks, beaker, measuring cylinder
- Conical flasks, test tube, pipette
- Flammable chemicals are ones which can
- Catch fire easily
- Explode easily
- Poison ayou
- Burn your skin
- Kipps apparatus is used in the laboratory for
- Obtaining pure gases
- Drying gases
- Obtaining continuous supply of gases
- Separation of gases
- A pipette is used for
- Measuring distance of length
- Measuring specific volume of liquids
- Measuring volumes
- Heating liquids
- Which of the following sets of apparatus are suitable for measuring volume of solutions
- Burette, Pipette, and beaker
- Burette, pipette and conical flask
- Measuring cylinder, burette and pipette
- Burette, flat bottomed flask and pipette
2. (a)Match the mixtures in List A with the methods of separation in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| LIST A | LIST B |
|
|
(b)Fill in the blanks below
- An exercise book is a matter because it has __________ and _________
- A change in the appearance and shape of a substance is a chemical change _____
- When water is heated it changes into vapor, when vapor is cooled it changes into
- A common example of matter that can exists in all three states is __________
- The particles in ____ are arranged closely and packed in fixed pattern.
SECTION B (80 Marks)
Answer all questions in this section
3. (a)Give the reason to why the chemicals in the laboratory should be labelled and well closed after use.
(b)the shelves in the laboratory should be labelled and constructed in a way that it is at the eye level and not above the eye level. Give the reason behind the statement
4. (a)Write the 6 scientific procedure in a form of descending (From the last to the first)
(b)There is no solution of a problem without research. Prove the statement
5. (a)What are the effect if one fails to follow the following laboratory instructions
- Failure to follow instructions guided
- Improper disposal of broken glass materials
(b) Write the functions of the following laboratory tools 2 functions
- Round bottomed flask
- burette
6.
- Mention three branches of science
- Who is a chemist?
- List 5 places where chemistry is applied
- Name three cleaning agents made by chemistry knowledge
7. Mention four importance of studying chemistry
8. (a) Mention two items used in each of the following categories that are made through application of chemistry
- Agriculture
- Pharmaceutical
- House hold items
- Food and beverages
- Transport
(b) Chemistry has contributed to environmental hazards. Briefly explain how.
9. (a)Define the following terms
- Laboratory
- First aid
- First aid kit
- Matter
(b)Mention 5 Laboratory rules
SECTION C
10. (a)What is first Aid?
(b) What does first Aids help? (Mention 5 point)
FORM ONE CHEMISTRY EXAM SERIES 120
FORM ONE CHEMISTRY EXAM SERIES 120
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY FORM ONE
TIME: 2 HOURS NOVEMBER 2022
INSTRUCTIONS
1. This paper Consist of sections A, B and C with total of 10 questions
2. Answer all questions in both sections
3. All writings should be in blue/black ink
4. All diagrams should be drawn in pencil
5. Write your assessment number at the top right corner
SECTION A (15Marks)
1. For each of the items (i)-(x), choose the correct answer from among the given alternative and write its letter in the box provide.
- The welders prefers to use non luminous flame for their work simply because
(A)It is available (B) it is easy to transport
(C) produce very hot flame (D) can be made by kerosene
- Spatula in the laboratory is used for scoping what types of substances?
(A)Liquid and gases (B) solids and liquids
(C) Powdery and gases (D) Solids and powdery
- Why oxygen as one of the components of air Is unique?
(A)It has ability to burn (B) it support combustion
(C) it is diatonic gas (D) combine with carbon dioxide
- Why it is necessary to boil drinking water?
(A) To make it tasteless (B) To remove impurities
(C) to kill micro-organism (D) To make it taste less
- How do chemists refer to a mixture of milk and water?
(A)Miscible solution (B) Suspension (C) Immiscible solution (D) Emulsion
- How can one prevent rusting in fragile instruments like camera?
(A)By galvanization (B) By using silica gel (C)By using oil (D) By using ethanol
- Which of the following is not man-made product of applying chemistry?
(B) Sugar (B) Fertilizer (C) Milk (D) Vaccines
- li>Spatula in the laboratory is used for scoping what types of substances? (A)Liquid and gases (B) solids and liquids
(C) Powdery and gases (D) Solids and powdery
- Fractional distillation is possible if the mixture constituents differ in _______
A. Boiling points
B. Melting points
C. Vaporing points
D. Freezing points
- Which of the following is NOT among the composition of air?
A. Noble gases
B. Hydrogen
C. Carbon dioxide
D. Nitrogen
2. Match the descriptions in List Awith their corresponding answer in List Bby writing the letter of the correct response beside the item number in the answer booklets (s) provided.
SECTION B: (70 Marks)
Answer all questions in this section
3. (a) Suggest the best method of separating the following mixtures:
i. Ethanol and water__________________________________________
ii. Sodium chloride and water___________________________________
iii. Green solution form leaves___________________________________
iv. Sand from ice_____________________________________________
v. Iron feelings and calcium carbonate powder____________________
b) i. Why is zinc used as a coat for iron and not vice versa?
ii) Is water a mixture or a compound? Give two reasons to support your answer.
4. (a) Mrs. Msema kweli was addressing the villagers about use of water for economic values. What 4 values will she tell the villagers
(b) Is an air a mixture or a compound? Give 4 reasons
5. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv. Sterile gauze
6. (a)Distinguish the following substances.
- Homogenous mixture from heterogeneous mixture.
- Miscible from immiscible liquids.
- Saturated from unsaturated solution.
(b)Give two chemical tests for pure water
7. (a) Water exists in three states of matter with two examples, mention three states
of matter.
(b) The figure below shows the relationships among the three states of matter. Name the process involved in A, B, C, D, Eand F.
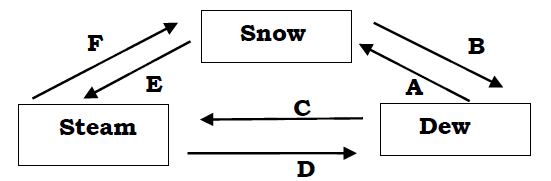
ii. Mention one (1) Chemical test for water
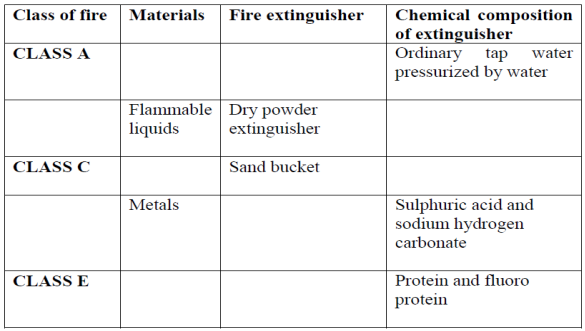
8. (a) Briefly explain the uses of the following;
- Oxygen gas
- Carbon dioxide gas
- Nitrogen gas.
(b) Complete the following table
9. (a) What should you do immediately if:
(i) The piece of broken beaker cuts your finger
(ii) Chemicals splash on your face
(iii) Your shirt has caught fire
(iv) Your fellow student swallows unknown chemical substance thinking that it was water
(b) Why do you think first aid is an important thing? Give two points:
SECTION B: 15 MARKS
10. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
FORM ONE CHEMISTRY EXAM SERIES 116
FORM ONE CHEMISTRY EXAM SERIES 116
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
FORM TWO NEW NECTA FORMAT
FORM ONE EXAMINATION – SEPTEMBER 2022
032 CHEMISTRY
TIME: 2:30 HOURS
INSTRUCTIONS
- This paper consists of sections A and B with a total of TEN (10) questions
- Answer all questions in the space provided.
- All writing must be in black or blue ink except for diagrams which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Zn= 65, X =32, O=16
SECTION A (15 Marks)
Answer all questions in this section
- For each of the items (i) - (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the bracket provided.
- The substance that can burn your skin is best described as: -
- Corrosive
- Explosive
- Flammable
- Toxic
- Oxidant
- When burette is used in the laboratory its calibration starts from above because.
- It measures the volume of remained
- It measures the used volume
- It measures the volume added
- It measures the volume of base added
- When burning a fuel produces blue color it means there is
- Adequate supply of oxygen with production of soot
- In adequate supply of oxygen with production of soot
- Adequate supply of oxygen with production of less heat
- Adequate supply of oxygen with production of more heat
- One of the following apparatus is used to measure fixed volume of liquid
- Burette
- Pipette
- Conical flask
- Measuring cylinder
- Volumetric flask
- What should be done if the results obtained from an experiment do not support the hypothesis?
- The results should be left out
- A new problem should be identified
- The experiment should be changed
- Ideas for further testing to find a solution should be given
- The hypothesis should be accepted
- Water exists in three forms, which are solid, liquid and vapour. Which among of the following are examples of liquid form of water
- Rain, snow and hail
- Dew, rain and ice
- Mist, steam and clouds
- Rain, hail, ice
- Rain, mist, and dew
- Emulsion is the mixture of liquids that do not completely mix with each other.
Which of the following set represent emulsion?
- Butter, lotion, toothpaste and milk
- Milk, gel, foam, sponge
- Cream, gel, lotion and paint
- Sponge, milk, smoke, insecticides
- Smoke, milk, paint and body spray
- Domestic utensils made up of iron do rust as a result of the presence of
- Air and fire
- Water and oil
- Air and water
- Air and oil
- Water and fire
- When Mr Mwendakwao took Glucose and dissolved in water to form a uniform mixture.
- water is solution, glucose is solvent and the product is solute
- water is solute, glucose is solvent and the product is solution
- water is solvent, glucose is solute and the product is solution
- water and glucose forms immiscible mixture
- water and glucose form emulsion
- A simple proof that some chemical reactions take place in our bodies is that
A. We eat a balanced diet
B. Doctors tell us so in the hospitals
C. We occasionally fall sick
D. The food we eat or the drinks we take are quite different from the waste products from our bodies
E. There is no proof
2. Match the items in List A with the responses in List B by writing the letter of the correct response beside the item number in the answer sheet or booklet provided.
| List A | List B |
| A. Water is used for extinguishing B. Oxygen and water/moisture C. Presence of Oxygen D. Fire triangle E. Presence of carbon dioxide F. Rusting G. Galvanization H. Class B fire I. Nitrogen |
SECTION B (70 MARKS)
ANSWER ALL QUESTIONS
3. (a) Consider the diagram below;
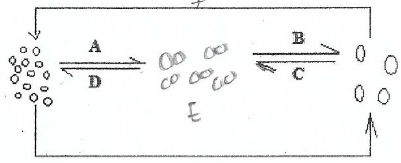
(i) Give aim of the above process
(ii) Identify the process A to F
(iii) Give two importance of the above diagram to our daily life.
(b) State whether the following is permanent change or temporary change,
(i) Dissolution of salt in water
(ii) Rotting of mangoes
4. (a) In which other areas do we find the warning signs out of laboratory (give four point)
(b) Explain how measurements of volume differ when using measuring cylinder and burette
(c) It is recommend that laboratory apparatus should be properly washed or wiped after use, explain the significance for this when
- Measuring volume of liquids
- Measuring mass of substance
5(a) Fill the blanks
- The techniques used to separate serum from blood samples is called _________________________
- The insoluble substances remain in a filter paper during filtration are termed as ___________________________
- Boiling points of substance reflect the strength of __________________
- The sub atomic particles of an atom are ____________________ and _____________________
- Solar energy is example of _______________________resources
(b) Suggest the best method of separating the following mixture
- Alcohol and water
- Sodium chloride and water
- Green solution from leaves
- Sand from rice
- Iron fillings and powder calcium carbonate
6. (a) Mrs. Janeth bought all necessary building materials as he wanted to build modern and expensive house for his mother, four months later he found out that almost all nails and Iron sheets have been rusted as she ignored leakage and small holes in the storage room, unfortunately he has no extra money to buy new nails and Iron sheets. As a chemistry student what will you advice Mrs. Janeth?
(b) Suppose you are in a bus traveling from Moshito Rombo for annual holiday, a bus driver went to the petrol station to fill fuel but he forgets to switch off the bus engine, this caused explosion and fire started. As a form four students:
(i) Will you be able to use fire extinguisher to fight against fire?
(ii) Which type of extinguisher will be suitable for you to use? Give reason of your choice
7. (a) Assign each of the properties to either luminous or non-luminous flame by putting a tick (?) on the respective column in the following table.
| Property of Flame | Luminous Flame | Non- luminous Flame |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b) Assume that you are doing an experiment in the laboratory at 07.30 pm and suddenly the lights go off. Give two reasons to justify the fact that you would consider luminous flame rather than non-luminous flame as an alternative source for lighting.
(c) Identify two properties of the flame produced by the Bunsen burner (air holes full opened) that can not be found in the flame produced by the spirit burner.
8. (a)Giving a reason, state whether rust will form or not in each of the situations (i) - (vi).
- Iron bar is dipped into boiling water.
- Painted iron bar is dipped into un-boiled water.
- Iron bar is dipped in un-boiled water.
- Oiled iron bar is left outside the room over nights.
- Alluminium wire is dipped in un-boiled water.
- A dry iron bar is wrapped with cotton wool.
(b)Briefly explain any four methods of preventing rusting.
9. (a)Why is petrol not recommended to be used as fuel in school laboratories? Briefly explain.
(b) Which three heat sources can be used to boil some water in the laboratory instead of the Bunsen burner?
(c) Arrange the following steps for lighting the Bunsen burner in a correct sequence using letter
A to F.
- Turn the collar to close the air hole completely.
- Turn on the gas fully to ensure that plenty of gas is entering the burner.
- Connect the Bunsen burner to the gas mains.
- Adjust the gas tap until the supply of gas is enough for a time.
- Light the gas at the top of the barrel with a lighted matchstick.
- Close the air hole.
SECTION C (15 MARKS)
10.(a) Identify the five accidents which are common in the laboratory and in each explain possible causes and preventive measures to be taken.
(b). Briefly explain the five classes of fires based on the nature of the burning material and the extinguisher required. Give one example for each class.
(c) Differentiate metals from non metals
FORM ONE CHEMISTRY EXAM SERIES 103
FORM ONE CHEMISTRY EXAM SERIES 103
THE PRESIDENTS OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM ONE-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- Chemistry helps us to explain
- Why and how matter undergoes changes
- What medicine to use when are fall sick
- How chemicals are used in agriculture
- How pesticides help in crop population
- A simple proof that chemical reactions takes place in our bodies is that
- We occasionally fall sick
- Doctors tell us in the hospital
- The food we eat or drinks we take are quite different from the waste products from our bodies
- We eat a balance diet.
- Which of the following is not a list of products of application of chemistry?
- Tables, chairs and bricks
- Shoes polish, antiseptics and toothpaste
- Soap, medicinal and perfume
- Fertilizer, pesticides and herbicides
- Chemistry is a scientific activity because
- Chemistry is studies in schools
- Its an interesting subject
- Knowledge of chemistry is acquired through observation
- Knowledge of chemistry is acquired through experiment and logical reasoning
- A condition in which the lungs are unable to get enough oxygen is known as
- Shock
- Fainting
- Suffocation
- Choking
- Choking can be treated by
- Cardio pulmonary resuscitation
- Expose the victim to the air
- Perform Heimlich manoeuvie
- Put a victim in the recovery position
- If the poison is on the skin then
- Do not apply any ointment
- Move the person to where there is a fresh air
- Give the victim fluids or juice
- Give on oral rehydration drinks
- A careless student secretly tied to mix up different chemicals just out of curiosity. The most thing that could happen to the student was to
- Damage the school properly
- Be expelled from school
- Annoy the teacher
- Produced poisonous cases that could lead to death
- Which of the following is not true about First Aid?
- First aid avoids an already bad situation from worsening
- First and helps to reduce pain, brings hope and encouragement
- Every student should be trained on how to administer first aid
- It is given to an accidental victim before seeking the doctors advice
- What is the use of splints first aid?
- Used for cutting bandages or pieces of cloth
- Used for holding pieces of bandages or cloth together
- Used to support broken bones and is tied using bandages
- Used for picking up and holding things
2. Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
(b) Answer the given questions by writing the correct answer in the blank spaces provided.
(i) In which stage is the hypothesis tested during scientific investigation? . . . . . . . . . . . . . . . . .
(ii) On which factor does the physical state of a molecule depend? . . . . . . . . . . . . . . . . .
(iii) Which properties depend on the proportions of mixing substances in a mixture? . . . . . . . . . . . . . . . . .
(iv) What are the building blocks of matter? . . . . . . . . . . . . . . . . .
SECTION B: 56 Marks
Answer all questions
3. Fill in the blank space
- What is a flame? ________________________________________________________________________________________________________________________________________________________________________________________
- What is a heat? ________________________________________________________________________________________________________________________________________________________________________________________
- Name the type of the flame that is produced when the air hole of the Bunsen burner are half closed __________________________________________________________________
- What causes the striking back of a flame in the Bunsen burner.
Mention only three causes
- __________________________________________________________________________
- __________________________________________________________________________
- __________________________________________________________________________
- Mention two ways which striking back is recovered
- _____________________________________________________________________________
- _____________________________________________________________________________
4. Study the figure below and answer the question that follows:
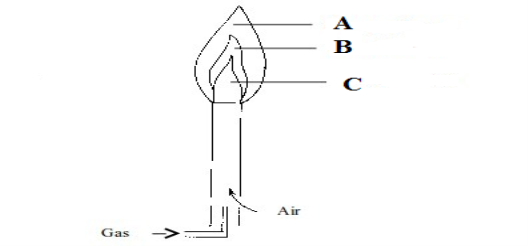
- Name the type of the flame indicate by the figure below _______________________________________________________
- Name the parts labeled: A _________________________________ B _________________________ C________________________________________
- What would happen if the march stick was placed in the part labeled C ____________________________________________________________________________________________
5. (a) List down ten laboratory rules
(b) With the help of diagram explain how each of the following lab apparatus works
(i) Condenser (ii) Burette (iii) pipette (iv) deflagrating (v) thermometer
(c) State two aims of the alchemists
(i) _____________________________________________________________________________
(ii) _____________________________________________________________________________
- What is the use of the following in the lab?
- Dark room _______________________________________________________________________
- Large windows __________________________________________________________________
- Circuit breaker __________________________________________________________________
6. Explain how chemistry is applied in the following field?
i)Communication ________________________________________________________________________________________________________________________________________________________________________________
ii)Transportation ________________________________________________________________________________________________________________________________________________________________________________
b)List down three common accidents that to occur in the chemistry lab and their possible causes
- ________________________________________________________________________________________________________________________________________________________________________________
- ________________________________________________________________________________________________________________________________________________________________________________
- ________________________________________________________________________________________________________________________________________________________________________________
c)Explain how you would administered first aid to a person having an electric shock at least four steps ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
7. With the help of a well labeled diagram of the Bunsen burner describe how the Bunsen burner work (the process involving lighting of the Bunsen burner)
8. With the help of a well labeled diagrams differentiate between the luminous flame and non luminous flame
9. With the aid of the diagram explain any four chemical signs that are found in the chemistry lab. Suggest their precaution measures.
FORM ONE CHEMISTRY EXAM SERIES 91
FORM ONE CHEMISTRY EXAM SERIES 91
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM ONE- MARCH/APRIL-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
SECTION A (20 Marks)
Answer All questions in this section.
- The term chemistry means;
- Study of nonliving things
- Study of physical properties of matter
- Study of drugs
- Study of composition and decomposition of matter
- Substances studied in chemistry are made up of;
- Compounds
- Matter
- Chemicals
- Element
- Which is not an importance of studying chemistry;
- Understand how matter undergo chemical change
- Make various medical products
- Understand the use of chemicals in agriculture
- Help us enter into various careers.
- A mother at home can apply chemistry to;
- Weed flowers in the garden
- Dusting the floor of her house
- Weighing maize flour
- Cooking food in the kitchen
- Which of the following illustrates a chemical reaction taking place in our body
- Falling sick
- Digestion
- Respiration
- Salivating
- Which of the following is not a list of application of chemistry?
- Table, chairs and Bricks
- Shoe polish, antiseptics and toothpaste
- Soap, medicinal drugs and perfumes
- Fertilizer, pesticides and herbicides
- Which of the following careers does not require the knowledge of chemistry?
- Medicine
- Agriculture
- Archeology
- Pharmacy
- Identify the skill not acquired during chemistry study
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Individuals who studied chemistry long time ago were called
- Alchemists
- AL chemistry
- Witch doctors
- Chemists
- Chemistry is list related to;
- Biology
- History
- Physics
- Geography
- MATCHING ITEMS QUESTIONS.
| LIST A | LIST B |
|
|
- Write T for true statement and F for a False statement.
- There are only two branches of science
- Chemistry has little application in our life
- Through chemistry, development of modern technology is made possible
- We only study chemistry to enter into careers
- Chemistry is only studied by chemists
b) Fill in the spaces below;
i) The field of science that deals with crop production and livestock management is called……………
ii) Ink, shoe polish, paper, clothes and rubber are examples of things produced from application of knowledge of…………………………………………………….
iii) Study of chemistry prepares people for different skills needed in our daily life such as………….
iv) Chemicals used to kill insects are called………………………………………………………..
v) The study of matter and its properties is called…………………………………
- A) mention three branches of science
b) Who is a chemist?
c) List 5 places where chemistry is applied
d) Name three cleaning agents made by chemistry knowledge
- Explain how chemistry is applied in the following;
- Health
- Agriculture
- Energy production
- Transport
- Modern technology
- Mention four importance of studying chemistry
- Mention two items used in each of the following categories that are made through application of chemistry
- Agriculture
- Pharmaceutical
- Household items
- Food and beverages
- Transport
b) Chemistry has contributed to environmental hazards. Briefly explain how
- a) Who are alchemists?
b) What was the aim of alchemists?
c) Briefly explain how chemistry has contributed to good life
- a) Mention areas where chemistry is applied.
b) Mention five careers one can enter by studying chemistry.
- a) mention two branches of chemistry
b) Differentiate chemistry from physics.
FORM ONE CHEMISTRY EXAM SERIES 79
FORM ONE CHEMISTRY EXAM SERIES 79
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY ANNUAL EXAMINATION
FORM ONE-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
SECTION A – (20 MARKS)
1. For each of the item (i)-(x) choose the correct answer from the choices given and write the corresponding letter in the table provided.
- Air entering the Bunsen burner is controlled by ___________(A) metal ring (B) air hole (C) metal jet (D) air ring
- Which of the following apparatus is used to measure a fixed volume of liquid? (a) burette (b) measuring cylinder (c) pipette(d) dropper
- A branch of science which deals with the study of matter and its properties is called ________ (a) meteology (b) astronomy (c) physics (d) chemistry
- Petrol is an example of _________ (a) corrosive substance (b) irritant substance (c) flammable substance (d) toxic substance
- First aid is provided to: (a) any person who is sick (b) an accident victim (c) an ill person (d) doctors and nurses
- A chemist should acquire the following skills except (a) experimentation (b) observation (c) problem identification (d) surgery
- One of the following is not laboratory rules. (a) heat flammable chemicals over open flame (b) turn off all burners when not in use (c) always wears protectors during experiment (d) never use broken glassware during experiment
- The chemical symbol lead is _________ (a)Nw (b) Pb (c) Ar (d) Sd
- Chemical spread on crops to destroy pests are called __________ (a) insecticide (b) pesticides (c) fertilizers (d) weed killers
- A pharmacist is a person who deals with ____________ a) chemical processing industries (b) medicine (c) laboratory experiment (d) agriculture
| I | ii | Iii | Iv | v | vi | vii | viii | ix | x |
|
|
|
|
|
|
|
|
|
|
|
2. a) Match each item in list A with the correct response in list B by writing its letter below the number of the corresponding item in the table provided
| LIST A | LIST B |
| A. hospital B. Vaccines C. Luminous flame D. deposition E. sublimation F. non luminous flame G. experimentation H. formulation hypothesis I. laboratory
|
| List A | I | Ii | iii | iv | v |
| List B |
|
|
|
|
|
b) fill the blanks provided
- Chemical used to kill insects are called ______________
- Are the people who study chemistry in ancient times ____________
- __________ is the study of nature
- The study of chemistry prepares people for different skills we need in our daily life such as _____________
- ____________ is anything which has mass and occupies a space
SECTION B (80 MARKS)
3 (a) Define chemistry __________________________________________________________________________________________________________________________
(b) Name two product in each of the following field made by application of chemistry
| Field | products |
| i) agriculture | a) |
|
| b) |
| ii) food and beverage industry | a) |
|
| b) |
|
|
|
c) mention four importance of studying chemistry
- ___________________________________________________________________________________
- __________________________________________________________________________________
- _________________________________________________________________________________
- _____________________________________________________________________________________
4. a) Define the following terms
- First aid _____________________________________________________________________________________________________
- First aid kit ___________________________________________________________________________________________________________________________________________________
- Fainting ____________________________________________________________________________________________________________________________________________
(b) Mention four importance of first aid to a victim
- _________________________________________________________
- _________________________________________________________
- ____________________________________________________________
- ________________________________________________________________
5. a) Draw and give one function of each of the following apparatus
| Name of apparatus | Drawing | functions |
| i) conical flask |
|
|
| ii) pipette |
|
|
b) mention four components of first kit and state their uses
- ________________________________________________________________________________
- ________________________________________________________________________________
- __________________________________________________________________________________
- ________________________________________________________________________
6. a) Mention three (3) state of matter and give three (3) examples of each state
- ________________________________________________________
- _______________________________________________________
- __________________________________________________________
b) what type of change are these
- Decaying of teeth _______________________________________________________
- Burning of charcoal _____________________________________________________
- Drying of wet clothes ____________________________________________________-
- Grinding piece of chalks ______________________________________________
- Ripens of fruits _______________________________________________________
- Sour milk ____________________________________________________________________
7. a) what is the differences between physical change and chemical change (4 points)
| PHYSICAL CHANGE | CHEMICAL CHANGE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b) The figure below shows the relationship among three states of matters: Name the process involved in A, B, C, D, E and F
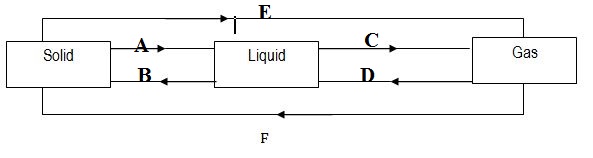
- Is____________________________________________
- Is_________________________________________________
- Is __________________________________________________
- Is_____________________________________________________
- Is_________________________________________________________
- Is __________________________________________________________-
8. a) i) what is flame? _______________________________________
ii) mention two types of flame produced by Bunsen burner
- ____________________________________________________________-
- ___________________________________________________________
b) Give four differences between the flames you have mentioned in 8 a (i) above
| i) |
|
| ii) |
|
| iii) |
|
| iv) |
|
9. What do you understand about the following terms
- Mixture____________________________________________________________________________________________________________________
- Solute _________________________________________________________________________________
- Solvent _____________________________________________________________________________________________________________________________
- Solution _____________________________________________________________________________
- Compound _____________________________________________________________________________________________________
10. i) Define an element______________________________________________________________________________________________________-
ii) write a chemical symbol for each of the following elements
- Iron _________________________________________________________________
- Copper __________________________________________________
- Silver _______________________________________________________
- Mercury _______________________________________________________
iii) write the name of the following element for each chemical symbols
- K_________________________-
- Ne__________________________
- Ca_______________________
- Pb __________________________-
FORM ONE CHEMISTRY EXAM SERIES 72
FORM ONE CHEMISTRY EXAM SERIES 72
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM ONE- AUG/SEPT 2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
(i) Air entering the Bunsen burner barrel can be controlled by
- metal ring
- air hole
- metal jet
- air ring
(ii) The appropriate extinguisher used to put off fire caused by cooking oil is:
- Water extinguisher
- Carbon extinguisher
- Wet chemical extinguisher
- Dry air extinguisher
(iii) A non-luminous flame is obtained if the hole is:
- fully opened
- partially opened
- closed
- half opened
(iv) Which of the following components can be separated by filtration method?
- Sand and water
- Kerosene and water
- Ethanol and water
- NaCl and water
(v) All domestic utensils made of iron undergo rusting when exposed to
- Air and fire
- Air and oil
- Air and water
- Water and oil
(vi) A change from gaseous state to solid state without passing through a liquid state is called:
- Deposition
- Sublimation
- Condensation
- Solidification
(vii) When a burning fuel produces blue colour it means there is:
A. adequate supply of oxygen with production of soot
B. inadequate supply of oxygen with production of more heat.
C. inadequate supply of oxygen with production of soot.
D. adequate supply of oxygen with production of more heat.
(viii) Why is water a universal solvent?
- It is neither acidic nor basic than any other known liquid.
- It dissolves more substances than any other known liquid.
- It occurs naturally in all the three states of matter than any other liquid.
- It dissolves both organic and inorganic solutes than any other liquid.
(ix) Which of the following gives the correct meaning of air?
- Mixture of Nitrogen, Oxygen and dust particles.
- Mixture of Nitrogen, Oxygen and Carbon dioxide.
- Mixture of Nitrogen, Oxygen and Water vapour
- Homogenous mixture of gases.
(x) Which of the following is the best apparatus for measuring accurately the volume of a given solution?
- Measuring cylinder
- Burette
- Beaker
- Conical flask
2. (a)Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| LIST A | LIST B |
|
|
(b) Answer the given questions by writing the correct answer in the blank spaces provided.
- The smallest particle of matter……………….
- Used to prepare a solution with known concentration…………..
- Preventing rusting by covering with zinc………………………
- The most abundance gas in air…………………..
- Turns lime water milky………………
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) Mariam was preparing food for her family using hot oil in a frying pan. Accidentally the pan tripped over and a huge fire spread over her kitchen floor.
(i)Mention two extinguishers which would be appropriate for putting out the fire.
(ii)Which fire extinguisher would be dangerous to use when trying to put out the fire in (a) above? Give reason. (b) Mention three conditions for a fire to start.
(c) (i) What is combustion?
(ii) Give three areas where combustion is applied.
4. (a) List down four careers that are a result of studying Chemistry. ![]()
![]() The following are possible causes of accidents which can occur in the Chemistry laboratory. State how you can avoid them.
The following are possible causes of accidents which can occur in the Chemistry laboratory. State how you can avoid them.
(i)Poisonous chemicals left in an unlocked cupboard .........
(ii)A student picking up a bottle containing concentrated H2S04 acid by the neck .........
(iii)Concentrated acids stored in the upper most shelf of cupboard
5.(a) Differentiate between:
(i)an atom and an element
(ii)Combustion and rusting
(iii)a solute and a solvent
(iv)a compound and a mixture
(b)Give two applications of chemistry in everyday life
(c)Why most laboratory apparati are made of glass?
6. Identify the five accidents which are common in the laboratory and in each explain possible causes and preventive measures to be taken.
7. (a) Why candles are not suitable for heating in the laboratory? Give two reasons.
(b) Differentiate luminous from non-luminous flame by giving five points.
8. (a) State one use of each of the items (i) – (x) in administering First Aid.
| S/N | Item | Use |
| (i) | Soap | |
| (ii) | Bandage | |
| (iii) | Sterile gauze | |
| (iv) | Iodine tincture | |
| (v) | Petroleum jelly |
(b) Effective use of the four senses of observation is important before a chemist can make conclusion. With four points, show how the senses are used as tools of observation during experimentation by giving one example for each.
(i) ………… ………… ……………
(ii) ………… …………… ………….
(iii) ………… ……………… …… ….
(iv) ………… …………… ……………
9. Briefly explain the five classes of fires based on the nature of the burning material and the extinguisher required. Give one example for each class.
10. Define the following terms:
(i) Brownian motion
(ii) Compound
(b) Identify whether the following is a physical or chemical change:
(i) Cutting aluminium foil into pieces
(ii) Lighting a match .
(c) How can you separate the following mixtures? Briefly explain.
(i)Water and kerosene
(ii)Salt and water
(iii)Ethanol and water
FORM ONE CHEMISTRY EXAM SERIES 64
FORM ONE CHEMISTRY EXAM SERIES 64
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRYTERMINAL EXAMINATION
FORM ONE-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- The following skills acquired through studying chemistry except;
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Chemistry is a scientific activity because;
- Chemistry is studied in schools
- Chemistry is interesting subject studied through data collection and analysis.
- Knowledge of chemistry is acquired through observation, experimentation and logical reasoning.
- It involves study of living and non living things.
- The following are laboratory rules except;
- Turn off water and gas tap after use
- Test any unlabelled substance before using it in the laboratory.
- Put off flames which are not in use
- Remove obstacles from gang ways.
- Splints are among of the first aid kit equipments which of the following sentences set describes its use;
- Used to pick up and holding things
- Used for cutting bandages or pieces of clothes
- Used to support broken bones and is tied using bandages.
- Used for holding pieces of bandages or cloth together.
- The Bunsen burner is likely to contain soot when;
- The air hole is closed
- The air hole is open but not fully
- The air holes of Bunsen burner are completely open.
- The burner is not raised
- The gas supply is high
- Which of the following takes place in experimental stage?
- Rejection of hypothesis
- Acceptance of hypothesis
- Concluding the scientific findings
- Carry out investigations
- In scientific procedure, what would be the next step to follow after experiments?
- Data interpretation
- Conclusion
- Observation
- Investigation
- In her first experiment, Mwajuma dissolved sulphuric acid in water and heat was evolved. In her second experiment he dissolved sugar in water and no heat was evolved or absorbed she conducted that;
- Her first experiment was physical change
- Both of her experiments were physical change
- Her second experiment was chemical change
- Her first experiment was chemical change
- In most general sense the word combustion means …………….
- Burning substance in oxygen or air.
- Burning compound of carbon in air.
- Burning any fuel in air or oxygen
- By chemical combination accompanied by light and heat in which or more reactants is gaseous
- 10 grams of copper powder were placed initiation tube containing air. The tube was very strongly for half an hour. At the end experiment;
- Copper increased in mass in changed colour
- Copper melted into liquid copper form.
- The total mass of tube and its content decreased.
- Water vapour appeared in the tube.
2. (a) Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
(b) Fill the following gaps with the correct answer
- Mixture of solute and solvent…………………………………………
- A box for keeping items to be used for first Aid…………………………….
- Solid obtained after filtration……………………………………..
- An apparatus used to measure fixed volume of a liquid………………..
- The smallest particle of matter…………………………………..
- (a) Define the following terms;
- Combustion ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
- Combustible material ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Label the parts marked A, B, C, D, E and F in the diagram below;
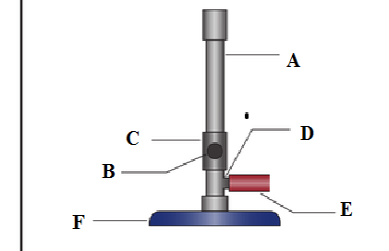
- ………………………………………..
- ………………………………………..
- ……………………………………….
- ………………………………………..
- ………………………………………..
- ………………………………………..
(c) What is the importance of parts labelled?
B ………………………………………………………………………………………
C ………………………………………………………………………………………
D ……………………………………………………………………………………….
- Fill the suitable word in the space provided;
- Bronze is an alloy formed by mixing ……………… and ……………………….
- Steel is an alloy formed by mixing ………………… and ……………………….
- …………………… is a solution in which like solvent used is not water.
- …………………….. is used to stir and mix substance uniformly.
- (a) Define the following terms and give one example in each case;
- A chemical symbol ……………………………………………………………………………………………………………………………………………………………………………………
- An element ……………………………………………………………………………………………………………………………………………………………………………………
- A compound ……………………………………………………………………………………………………………………………………………………………………………………
- Suspension ……………………………………………………………………………………………………………………………………………………………………………………
(b) Write names of elements represented by the following symbols
i. Au ……………………………………
ii. Ag …………………………………….
iii. Hg ……………………………………..
iv. P ………………………………………..
v. Sn …………………………………………
- Draw apparatus which are used to perform the following process in the laboratory;
| Process | Apparatus diagram |
| |
| (b)A flat apparatus at the base used to hold volume of liquid during experiment. | |
| © Wire placed on top of tripod stand. | |
| (d)Used for scooping small quantities of powder or crystalline chemicals. | |
| (e)Used for holding heating and mixing liquid – it measures estimate volume of liquids. |
- (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
(b) Distinguish between the following;
i. Hypothesis against fact ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii. Deposition against sublimation ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Point out any 6 differences between metals and non – metals ;
| Metals | Non metals |
- (a) Briefly explain any five methods of preventing rusting.
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
(b) A certain pink coloured compound is heated to form blue colour. When water is
added to sample , it changed back to pink.
i. What change is demonstrated by the compound …………………………………....
ii. Explain the reasons for your choice.
…………………………………………………………………………………………………………………………………………………………………………………………...….
(c) State the type of changes demonstrated by the following phenomenon;
- Burning of firewood ……………………………………………………….
- Rotting of meat …………………………………………………………….
- Heating frying pan …………………………………………………………..
- Melting of butterfat …………………………………………………………
- Change of cloud to rain ……………………………………………………..
- Heating of iron rod …………………………………………………………..
- Your provided with the diagrams below, study it carefully and answer questions that follows;
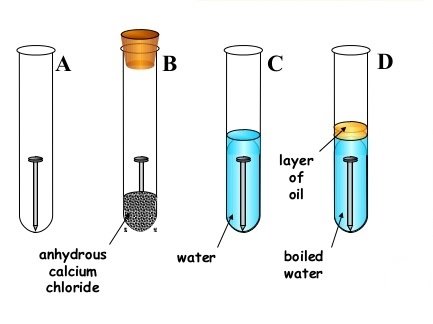
- State short and clear description about what will be observed in each test tube A – D after 2 or 3 days;
- Test tube A. ………………………………………………….
- Test tube B …………………………………………………..
- Test tube C. …………………………………………………..
- Test tube D. …………………………………………………..
- Why the water in the test tube D boiled the covered with oil. ……………………………………………………………………………………………………………………………………………………………………………………
- What is the function of calcium chloride in test tube B? …………………………………………………………………………………………………………………………………………………………………………………….
- What conclusion can you draw from your experiment? …………………………………………………………………………………………
- (i) Give two similarities between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Give two differences between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
FORM ONE CHEMISTRY EXAM SERIES 55
FORM ONE CHEMISTRY EXAM SERIES 55
Student’s Examination No..............................................
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION-MARCH
FORM ONE-2021
Time: 2:30 Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(x), choose the correct answer from the alternatives given
- Kipp’s apparatus is used in the laboratory for;
- Drying gases
- Obtaining continuous supply of gases
- Obtaining pure gases
- Separation of gases
- Chemical which can easily catch fire are best termed as;
- Flammable
- toxic
- explosive
- corrosive
- A pipette is used for;
- Measuring distance of length
- Measuring specific volume of liquid
- Measuring volume
- Heating liquid
- Which of the following sets of apparatus are suitable for measuring volume of solution or liquids?
- Burette, pipette and beaker
- Burette, pipette and conical flask
- Measuring cylinder, pipette and burette
- Burette, flat bottomed flask and pipette
- The substance that can burn your skin is best describe as;
- Flammable
- corrosive
- explosive
- toxic
- Which of the following warning signs is likely to be found on a bottle containing petrol;
- Oxidant
- flammable
- corrosive
- irritant
- Which of the following chemical warning signs is likely to be found on a bottle containing potassium permanganate(vii);
- Oxidant
- corrosive
- explosive
- toxic
- Which of the following warning signs is likely to appear on the bottle containing concentrated Nitric Acid in the laboratory;
- Corrosive
- explosive
- flammable
- irritan
- Consider the diagram below of a certain apparatus in the laboratory;
Which is the correct reading of the above measurement?
- 10.50cm³
- 10.5cm³
- 10.60cm³
- 10.6cm³
- A student who get burns accidently in the chemistry laboratory would be given one of the following as a first Aid;
- Potassium permanganate
- Nitric acid
- petroleum jelly
- antibiotic solution
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
2. You are provided with two list A and List B. choose a word(s) from list B which matches the statement or phrase in list A and write down its letter;
| LIST A | LIST B |
|
|
| List A | i | ii | iii | iv | v |
| List B |
SECTION B
3. (a) Define the following terms;
- First Aid
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- First Aid Kit
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Laboratory apparatus
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Why beaker is used to estimate the volume of liquid and not to measure exactly volume of
liquid?
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- (a) Give two reasons why most of laboratory apparatus are made up with the glass materials
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Why laboratory should have larger window?
.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- List down three causes of accidents in the scene;
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Suggest how each danger would be removed;
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Explain the First Aid procedures for the person with severe bleeding;
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- ... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- (a) Laboratory apparatus are written the word “PYREX” What does mean by the above term . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
b)Draw well diagram and state one function of the following apparatus.
- Pipette
- Burette
- Deflagrating spoon
- Beaker
- Measuring cylinder
- (a) Define the term “chemical warning sign”
.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b)Draw the chemical warning sign of the following substance;
- Methylated spirit;
- Insecticide
- Ammonia gas
- Concentrated sulphuric acid
- Potassium nitrate
- Write what might happen if you never followed instruction;
- Always wash your hands with soap before you leave a laboratory;.
.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Always replace the cover (stopper) after using chemical
.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- (a) The following list contains the name of pieces of apparatus with the letter in each name JUMBLED UP. Rearrange the letters to produce the name of pieces of apparatus;
- LUPSTAA.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- TPTIPEE .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- GOTHUR.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- ROMTMREEHET .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- TERSTBEU.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- KSAFL.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- REBAKE .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Name two apparatus which can be used as container from above list;
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ..
(c)Name one apparatus which can be used to measure volume from 9 (a) above
.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- (a) Mention three laboratory rules after experiment;
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) List down three (3) item found in first Aid Kit for providing First Aid
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c)Mention four (4) importance of providing First Aid to the victim
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
THE END
BEST WISHES
FORM ONE CHEMISTRY EXAM SERIES 47
FORM ONE CHEMISTRY EXAM SERIES 47
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION-MARCH
FORM ONE-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(x), choose the correct answer from the alternatives given.
- Chemistry is one of the sciences which deals with
- Alkalinity and basicity of substance
- The study of body cells
- Composition, properties and behavior of matter
- Chemical changes
- In chemistry experiments test
- Data
- Problem
- Hypotheses
- Observation
- When a chemist studies a substance, he/ she is interested in its
- Force of attraction
- Shape
- Smell
- Properties
- A chemist should acquire all of the following skills except
- Experimentation
- Observation
- Problem identification
- Surgery
- A chemistry defined as
- The scientific study of matter, compound, and chemical reaction
- The scientific study of compounds, mixture and organic substances
- The scientific study of composition structure and properties of matter
- The study of relation between human being, medicine, and pollution
- A mother at home can apply chemistry to;
- Weed flowers in the garden
- Dusting the floor of her house
- Weighing maize flour
- Cooking food in the kitchen
- Which of the following illustrates a chemical reaction taking place in our body
- Falling sick
- Digestion
- Respiration
- Salivating
- Which of the following is not a list of application of chemistry?
- Table, chairs and Bricks
- Shoe polish, antiseptics and toothpaste
- Soap, medicinal drugs and perfumes
- Fertilizer, pesticides and herbicides
- Which of the following careers does not require the knowledge of chemistry?
- Medicine
- Agriculture
- Archeology
- Pharmacy
- Identify the skill not acquired during chemistry study
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Individuals who studied chemistry long time ago were called
- Alchemists
- AL chemistry
- Witch doctors
- Chemists
2. You are given LIST A and LIST B. Choose the best response from LIST B that matches with description from LIST A.
| LIST A | LIST B |
|
|
(b) Answer the following questions by writing the correct answer on the blank spaces provided.
i) The field of science that deals with crop production and livestock management is called . . . . . . . . . . . . . . . . . . . . . . . . . . .
ii) Ink, shoe polish, paper, clothes and rubber are examples of things produced from application of knowledge of . . . . . . . . . . . . . . . . . . . . . . . . . . .
iii) Study of chemistry prepares people for different skills needed in our daily life such as . . . . . . . . . . . . . . . . . . . . . . . . . . .
iv) Chemicals used to kill insects are called . . . . . . . . . . . . . . . . . . . . . . . . . . .
v) The study of matter and its properties is called . . . . . . . . . . . . . . . . . . . . . . . . . . .
SECTION B (80 Marks)
Answer all questions in this section.
3. Mention two items used in each of the following categories that are made through application of chemistry
- Agriculture
- Pharmaceutical
- Household items
- Food and beverages
- Transport
b) Chemistry has contributed to environmental hazards. Briefly explain how
4. (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Distinguish between the following;
i. Hypothesis against fact . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
ii. Deposition against sublimation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
5.(a) List down ten laboratory rules
(b) With the help of diagram explain how each of the following lab apparatus works
(i) Condenser (ii) Burette (iii) pipette (iv) deflagrating (v) thermometer
(c) State two aims of the alchemists
(i) _____________________________________________________________________________
(ii) _____________________________________________________________________________
6.Define the following terms;
- Laboratory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- First aid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- First aid kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Matter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7.Explain the causes of the following accidents in the laboratory;
- Shock
- Bleeding
- Skin burn
8.Mention five items found in first aid kit and their functions
9.(a) What do you understand by the term laboratory? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Mention three characteristics of a good laboratory
(i) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Why is it necessary to put on the following during the conduction of experiments
- Laboratory coat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Plastic goggles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
10.Name six injuries which can be caused by running in the laboratories or in the corridor of it.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
FORM ONE CHEMISTRY EXAM SERIES 44
FORM ONE CHEMISTRY EXAM SERIES 44
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY MID TERM EXAMINATION
FORM ONE-2021
Time: 2:30 Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(x), choose the correct answer from the alternatives given.
- Chemistry is one of the sciences which deals with
- Alkalinity and basicity of substance
- The study of body cells
- Composition, properties and behavior of matter
- Chemical changes
- In chemistry experiments test
- Data
- Problem
- Hypotheses
- Observation
- When a chemist studies a substance, he/ she is interested in its
- Force of attraction
- Shape
- Smell
- Properties
- A chemist should acquire all of the following skills except
- Experimentation
- Observation
- Problem identification
- Surgery
- A chemistry defined as
- The scientific study of matter, compound, and chemical reaction
- The scientific study of compounds, mixture and organic substances
- The scientific study of composition structure and properties of matter
- The study of relation between human being, medicine, and pollution
- A mother at home can apply chemistry to;
- Weed flowers in the garden
- Dusting the floor of her house
- Weighing maize flour
- Cooking food in the kitchen
- Which of the following illustrates a chemical reaction taking place in our body
- Falling sick
- Digestion
- Respiration
- Salivating
- Which of the following is not a list of application of chemistry?
- Table, chairs and Bricks
- Shoe polish, antiseptics and toothpaste
- Soap, medicinal drugs and perfumes
- Fertilizer, pesticides and herbicides
- Which of the following careers does not require the knowledge of chemistry?
- Medicine
- Agriculture
- Archeology
- Pharmacy
- Identify the skill not acquired during chemistry study
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Individuals who studied chemistry long time ago were called
- Alchemists
- AL chemistry
- Witch doctors
- Chemists
- You are given LIST A and LIST B. Choose the best response from LIST B that matches with description from LIST A.
| LIST A | LIST B |
|
|
(b) Answer the following questions by writing the correct answer on the blank spaces provided.
i) The field of science that deals with crop production and livestock management is called . . . . . . . . . . . . . . . . . . . . . . . .
ii) Ink, shoe polish, paper, clothes and rubber are examples of things produced from application of knowledge of. . . . . . . . . . . . . . . . . . . . . . . .
iii) Study of chemistry prepares people for different skills needed in our daily life such as. . . . . . . . . . . . . . . . . . . . . . . .
iv) Chemicals used to kill insects are called . . . . . . . . . . . . . . . . . . . . . . . .
v) The study of matter and its properties is called. . . . . . . . . . . . . . . . . . . . . . . .
SECTION B (80 Marks)
Answer all questions in this section.
3. Mention two items used in each of the following categories that are made through application of chemistry
- Agriculture
- Pharmaceutical
- Household items
- Food and beverages
- Transport
b) Chemistry has contributed to environmental hazards. Briefly explain how
4. (a) Mention and explain briefly any five warning signs found in chemical containers giving one example in each case.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Distinguish between the following;
i. Hypothesis against fact
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
ii. Deposition against sublimation
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
5.(a) List down ten laboratory rules
(b) With the help of diagram explain how each of the following lab apparatus works
(i) Condenser (ii) Burette (iii) pipette (iv) deflagrating (v) thermometer
(c) State two aims of the alchemists
(i) _____________________________________________________________________________
(ii) _____________________________________________________________________________
6.Define the following terms;
- Laboratory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- First aid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- First aid kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Matter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7.Explain the causes of the following accidents in the laboratory;
- Shock
- Bleeding
- Skin burn
8.Mention five items found in first aid kit and their functions
9.(a) What do you understand by the term laboratory? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Mention three characteristics of a good laboratory
(i) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ..
(ii) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Why is it necessary to put on the following during the conduction of experiments
- Laboratory coat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- Plastic goggles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
10.Name six injuries which can be caused by running in the laboratories or in the corridor of it.
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
FORM ONE CHEMISTRY EXAM SERIES 39
FORM ONE CHEMISTRY EXAM SERIES 39
THE PRESIDENTS OFFICE MINISTRY OF LOCAL GOVERNMENT AND REGIONAL ADMINISTRATION
MIDTERM EXAMINATION MARCH 2021
CHEMISTRY FORM I
1. Choose the best answer from the alternatives given and write its answer in the space provided.
- People who studied chemistry long time ago were called?
- Scientists
- Chemistry
- Chemists
- Alchemist
- Which is not a branch of science?
- Physics
- Biology
- Mathematics
- Geometry
- The study of matter in relation to energy is termed as
- Physics
- Geography
- Kinetics
- Chemistry
- Which of the following is chemistry knowledge not applied?
- In hospitals
- In industry
- In research institutions
- At homes
- A person who studies chemistry is called
- Chemistry
- Alchemist
- Chemist
- Physicist.
- Which of the field of study below does not need chemistry knowledge?
- Medicine
- Agriculture
- Vetinery medicine
- Geography
- Decomposition of matter is also termed as
- Building matter
- Consolidating matter
- Breaking up matter
- Synthesizing matter
- Which is not a feature of chemistry laboratory?
- Large windows
- Rough floor
- A bit dark
- Fitted with water supply
- Which is not an importance of studying chemistry?
- Help us enter into careers
- Through chemistry knowledge, we make several materials
- Chemistry knowledge help us to answer difficult questions
- Chemistry knowledge enable us understand our bodies well.
- An instrument used to measure fixed volume of liquid in laboratory is
- Burrete
- Beaker
- Pipette
- Measuring cylinder.
2. Match the correct answer in list A with alternative given in list B by writing the answer of the correct answer in the spaces provided.
| LIST A | LIST B |
|
|
3. Complete the following sentences with the correct answer.
- Breaking up of matter is called . . . . . . . . . . . . . . . . . . . . . . . .
- The study of organic compound falls into which branch of chemistry . . . . . . . . . . . . . . . . . . . . . . . .
- Help given to an accident victim before going to the hospital is called . . . . . . . . . . . . . . . . . . . . . . . .
- The word laboratory comes from Greek word . . . . . . . . . . . . . . . . . . . . . . . .
- An expected incidence that happens and results into injury is called . . . . . . . . . . . . . . . . . . . . . . . .
4. Explain five significance of studying chemistry
5. Mention five areas where the knowledge of chemistry is applied
6. (a) What is a laboratory?
(b) Mention six rules to follow while in laboratory.
7.(a) What are apparatus?
(b) State four materials used to make laboratory apparatus and for each case give an example.
8. describe five fields that are related to chemistry
9. State the uses of the following apparatus used in chemistry laboratory
- Wire gauze
- Beaker
- Conical flask
- Measuring cylinder
- Burette
10. Mention and explain five factors that cause accidents in laboratory.
FORM ONE CHEMISTRY EXAM SERIES 36
FORM ONE CHEMISTRY EXAM SERIES 36
PRESIDENT'S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ANNUAL EXAMINATION
CHEMISTRYFORM ONE
NAME………………………………………..CLASS……………………………TIME: 2HRS
INSTRUCTIONS:-
-This paper consists of three sections- A, AND B
-Answer all questions in section A and B.
SECTION A: 15 MARKS
- Choose the most correct answer from among of the given alternatives and write its letter in the space provided;
- The following skills acquired through studying chemistry except;
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Chemistry is a scientific activity because;
- Chemistry is studied in schools
- Chemistry is interesting subject studied through data collection and analysis.
- Knowledge of chemistry is acquired through observation, experimentation and logical reasoning.
- It involves study of living and non living things.
- The following are laboratory rules except;
- Turn off water and gas tap after use
- Test any unlabelled substance before using it in the laboratory.
- Put off flames which are not in use
- Remove obstacles from gang ways.
- Splints are among of the first aid kit equipments which of the following sentences set describes its use;
- Used to pick up and holding things
- Used for cutting bandages or pieces of clothes
- Used to support broken bones and is tied using bandages.
- Used for holding pieces of bandages or cloth together.
- The Bunsen burner is likely to contain soot when;
- The air hole is closed
- The air hole is open but not fully
- The air holes of Bunsen burner are completely open.
- The burner is not raised
- The gas supply is high
- Which of the following takes place in experimental stage?
- Rejection of hypothesis
- Acceptance of hypothesis
- Concluding the scientific findings
- Carry out investigations
- In scientific procedure, what would be the next step to follow after experiments?
- Data interpretation
- Conclusion
- Observation
- Investigation
- In her first experiment, Mwajuma dissolved sulphuric acid in water and heat was evolved. In her second experiment he dissolved sugar in water and no heat was evolved or absorbed she conducted that;
- Her first experiment was physical change
- Both of her experiments were physical change
- Her second experiment was chemical change
- Her first experiment was chemical change
- In most general sense the word combustion means …………….
- Burning substance in oxygen or air.
- Burning compound of carbon in air.
- Burning any fuel in air or oxygen
- By chemical combination accompanied by light and heat in which or more reactants is gaseous
- 10 grams of copper powder were placed initiation tube containing air. The tube was very strongly for half an hour. At the end experiment;
- Copper increased in mass in changed colour
- Copper melted into liquid copper form.
- The total mass of tube and its content decreased.
- Water vapour appeared in the tube.
| i | ii | iii | iv | v | vi | vii | viii | ix | x |
- Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
All materials used for preventing rusting aim at preventing water and oxygen from reaching the iron.Write T for correct statement and F for incorrect statement;
- Accidents always happen in the laboratory.
- All experiments must be done in the fume cupboards.
- Rusting cannot cause corrosive to some articles made up iron or steel.
- Desiccator is an apparatus used to dry solid chemicals.
SECTION B 80 MARKS
(a) Define the following terms;z
- Combustion ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
- Combustible material ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Label the parts marked A, B, C, D, E and F in the diagram below;
- ………………………………………..
- ………………………………………..
- ……………………………………….
- ………………………………………..
- ………………………………………..
- ………………………………………..
(c) What is the importance of parts labelled?
B ………………………………………………………………………………………
C ………………………………………………………………………………………
D ……………………………………………………………………………………….
- Fill the suitable word in the space provided;
(a)Bronze is an alloy formed by mixing ……………… and ……………………….
(b)Steel is an alloy formed by mixing ………………… and ……………………….
(c)…………………… is a solution in which like solvent used is not water.
(d)…………………….. is used to stir and mix substance uniformly.
- (a) Define the following terms and give one example in each case;
- A chemical symbol ……………………………………………………………………………………………………………………………………………………………………………………
- An element ……………………………………………………………………………………………………………………………………………………………………………………
- A compound ……………………………………………………………………………………………………………………………………………………………………………………
- Suspension ……………………………………………………………………………………………………………………………………………………………………………………
(b) Write names of elements represented by the following symbols
i.Au ……………………………………
ii. Ag …………………………………….
iii. Hg ……………………………………..
iv. P ………………………………………..
v. Sn …………………………………………
- Draw apparatus which are used to perform the following process in the laboratory;
| Process | Apparatus diagram |
| (a)It is used to measure accurate 20/25cm3 of liquid. | |
| (b)A flat apparatus at the base used to hold volume of liquid during experiment. | |
| © Wire placed on top of tripod stand. | |
| (d)Used for scooping small quantities of powder or crystalline chemicals. | |
| (e)Used for holding heating and mixing liquid – it measures estimate volume of liquids. |
- (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
i)………………………………………………………………………………………………………………………………………………………………………………
ii)………………………………………………………………………………………………………………………………………………………………………………
iii)………………………………………………………………………………………………………………………………………………………………………………
iv)………………………………………………………………………………………………………………………………………………………………………………
v)………………………………………………………………………………………………………………………………………………………………………………
(b) Distinguish between the following;
i.Hypothesis against fact ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii. Deposition against sublimation ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Point out any 6 differences between metals and non – metals ;
| Metals | Non metals |
- (a) Briefly explain any five methods of preventing rusting.
i)………………………………………………………………………………………………………………………………………………………………………………
ii)………………………………………………………………………………………………………………………………………………………………………………
iii)………………………………………………………………………………………………………………………………………………………………………………
iv)………………………………………………………………………………………………………………………………………………………………………………
v)………………………………………………………………………………………………………………………………………………………………………………
(b) A certain pink coloured compound is heated to form blue colour. When water is
added to sample , it changed back to pink.
i. What change is demonstrated by the compound …………………………………....
ii. Explain the reasons for your choice.
…………………………………………………………………………………………………………………………………………………………………………………………...….
(c) State the type of changes demonstrated by the following phenomenon;
- Burning of firewood ……………………………………………………….
- Rotting of meat …………………………………………………………….
- Heating frying pan …………………………………………………………..
- Melting of butterfat …………………………………………………………
- Change of cloud to rain ……………………………………………………..
- Heating of iron rod …………………………………………………………..
- Your provided with the diagrams below, study it carefully and answer questions that follows;
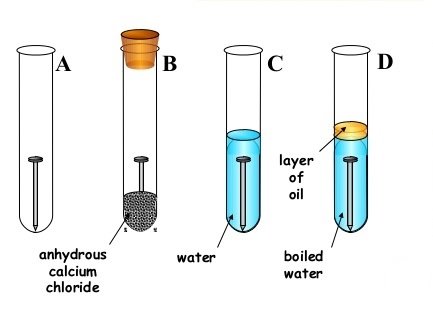
(a)State short and clear description about what will be observed in each test tube A – D after 2 or 3 days;
- Test tube A. ………………………………………………….
- Test tube B …………………………………………………..
- Test tube C. …………………………………………………..
- Test tube D. …………………………………………………..
(b)Why the water in the test tube D boiled the covered with oil. ……………………………………………………………………………………………………………………………………………………………………………………
(c)What is the function of calcium chloride in test tube B? …………………………………………………………………………………………………………………………………………………………………………………….
(d)What conclusion can you draw from your experiment? …………………………………………………………………………………………
(e)(i) Give two similarities between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Give two differences between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
FORM ONE CHEMISTRY EXAM SERIES 26
FORM ONE CHEMISTRY EXAM SERIES 26
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
AUGUST-SEPTEMBEREXAMINATION SERIES
CHEMISTRYFORM-1
2020
2:30 HOURS
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
- The following atomic masses may be used; H =1, N =14, O = 16, S = 32, Ca = 40
SECTION A (20 Marks)
Answer all questions in this section.
1.For each of the items (i) –(x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) What is the best way of keeping a clean test tube after use?
- Keeping it in water
- Keeping it on a test tube holder
- Keeping it in a basin for test tubes
- Keeping it on a test tube rack.
(ii) Which one of the following does not involve the processes of urban water treatment and purification?
- Sedimentation
- Distillation
- Filtration
- Chlorination.
(iii) Why hydrogen gas is not a constituent of air?
- Because of being water soluble
- Because of being denser than air
- Because of being very light
- Because of being highly flammable.
(iv) Which is the suitable alternative heat source to be used in absence of Bunsen Burner?
- Torch and spirit burner
- Torch and kerosene stove
- Kerosene stove and spirit burner
- Firewood and torch.
(v) What happens when substance A reacts with substance B to form a new substance C?
- Substance A and B are said to have formed a solution
- Substance A and B are said to have undergone a physical change.
- Substance A and B are said to have undergone a chemical change.
- Substance A and B are said to have undergone a dissolution.
(vi) Which components make fire triangle?
- Oxygen, fuel and heat
- Oxygen, nitrogen and heat
- Oxygen, fuel and carbon dioxide
- Oxygen, heat and hydrogen.
(vii) Which state is involved when drying wet clothes?
- Liquid to solid
- Solid to gas
- Gas to liquid
- Liquid to gas.
(viii) Why Non – luminous flame is the most applicable flame for heating purposes?
- It is very nosy
- It has no soot.
- It is very hot
- It has air holes open
(ix) Chemistry is a branch of Science which deals with:
- matter in relation to energy.
- matter in relation to decomposition.
- matter composition and its decomposition.
- properties of conservation of matter.
(x) The process of removing solid contaminants from water is known as:
- water decantation.
- water solidification.
- water purification.
- water sedimentation.
2. (a) Match each item in List A with a correct response in List B by writing the letter of the correct response below the corresponding item number in the table provided.
| List A | List B |
| (i)A solvent which dissolves most substances to form solution. (ii)A substance that has no definite shape or size. (iii)A substance that has a fixed shape and volume (iv)A substance whose components can be separated by physical means (v)Homogeneous mixture of two or more substances. |
|
(b) Fill in the blank spaces by using the appropriate terms
(i)Solid particles formed after filtration......................
(ii)Serum is separated from blood samples by employing a technique called………………..
(iii)Boiling points of substances reflect the strength of……………….
(iv)Grinding chalk into a powder involves changing the state of …………………….
(v) The liquid formed after filtration..........................
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) State one use of each of the items (i) – (x) in administering First Aid.
| S/N | Item | Use |
| (i) | Soap | |
| (ii) | Bandage | |
| (iii) | Sterile gauze | |
| (iv) | Iodine tincture | |
| (v) | Petroleum jelly |
(b) Give one function of each of the following apparatus in the chemistry laboratory.
(i) Spatula…………… …………… ……………….
(ii) Gas jar…………… ……………. …… ……….
(iii) Lie – big condenser …………… …… …………
(iv) Motor and pestle…… …………… ……………
(v) Wire gauze… ………… ………… …………….
4. (a) Differentiate hypothesis from analysis.
(b) Effective use of the four senses of observation is important before a chemist can make conclusion. With four points, show how the senses are used as tools of observation during experimentation by giving one example for each.
(i)………… ………… ……………
(ii)………… …………… ………….
(iii)………… ……………… …… ….
(iv)………… …………… ……………
5. What precautions will you take in handling chemicals having the warning signs shown in the table? Give two precautions in each sign.
| S/N | Sign | Relevant Precaution |
| a) |
| (i)………… ……… …………… …. (ii)………… ………… ……………. |
| b) |
| (i)…………… …………… …. (ii)………… ………… …… …. |
| c) |
| (i)…… …………… ……………. (ii)……… …………… …………. |
| d) |
| (i)…………… ……………… ……. (ii)…………… ………… …………. |
| e) | OXIDANT | (i)…………… ……… ……………. (ii)…………… …………… ………. |
6. Briefly explain the five classes of fires based on the nature of the burning material and the extinguisher required. Give one example for each class.
7. Explain three factors which affect the problem being investigated (b) Explain two areas where scientific procedure is applied.
8. Explain each of the following terms:
(i)Burn
(ii) Bruises
(iii)Fainting ..............................................
(b) What are the six procedures which can be used to help a person with severe bleeding on the wound?
9. Give three differences between the following:
(i)Physical changes and chemical changes
(ii)Mixture and compounds
10.(a) Define the following terms as applied in Chemistry:
(i)Flame
(ii)Bunsen burner
(iii)Laboratory
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
FORM ONE CHEMISTRY EXAM SERIES 18
FORM ONE CHEMISTRY EXAM SERIES 18
THE PRESIDENTS OFFICE
MINISTRY OF EDUCATION AND VOCATIONAL TRAINING
FORM ONE MID TERM EXAMINATION- MARCH 2020
CHEMISTRY
Duration: 2:30 Hours
INSTRUCTIONS.
- This paper consists of sections A and B with a total of ten (10) questions.
- Answer all questions in spaces provided.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number at the top right corner of every page.
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) (x), choose the correct answer from the among the given alternatives and write its letter in the box provide.
(i) Chemistry is one of the sciences which deal with.
- Alkalinity and basicity of substances.
- The study of body cells
- Composition, properties and behavior if matter.
- Chemical changes.
(ii) A pipette is sued for:
- Measuring distance of length
- Measuring specific volume of liquids
- Measuring volumes
- Heating liquid
(iii) The states of matter are:-
- Element, gas and mixture
- Liquid, moisture and element
- Water, moisture and solid
- Gas, liquid and solid.
(iv) In chemistry experiments test:
- Data
- Problems
- Hypotheses
- Observation
(v) Which of the following sets of apparatus are suitable for measuring volume of solutions?
- Burette, pipette and beaker.
- Burette, pipette and conical flask.
- Measuring cylinder, burette and pipette.
- Burette, flax bottomed flask and pipette.
(vi) The substance that can burn your skin is best described as:
- Flammable
- Corrosive
- Explosive
- Toxic
(vii) Sublimation is the process whereby:-
- Substances float in liquids when heated.
- A solid substance changes directly to vapour without the liquid states when heated
- A mixture of solid substances is separated by heating
- An alcohol is separated from water.
(viii) One of the following apparatuses is used to measure a fixed volume of liquids.
- Pipette
- Burette
- Measuring cylinder
- Beaker
(ix) Coloured substances can be separated through the process called?
- Filtration
- Chromatography
- Distillation
- Sublimation.
(x) Acid changes colour litmus paper form:
- Blue to yellow
- Red to blue
- Red to pink
- Blue to red
2. (a) You are provided with two lists, A and B. Choose a word(s) from list B which matches the statement in list and write its letter against the appropriate statement in the space provided.
| List A | List B |
| (i) Studied chemistry long time ago (ii) An intelligent guess (iii) Making sense of information (iv) Is used to measure fixed volume of a liquid (v) A Series of investigation aimed at discovering a fact. |
|
(b) Fill in the blank spaces by using the appropriate terms.
- Used in measuring mass .
- The person who studies chemistry .
- The people who studied chemistry long time ago
- Studies composition and decomposition of matter ..
- A proved hypothesis ..
SECTION B (80 Marks)
Answer all questions in this section.
3. (a) What is matter?
(b) Mention three (3) states of matter.
4. (a) Write the chemical symbols for the following
(i) Lead
(ii) Potassium
(iii) Gold
(iv) Sodium
(v) Chlorine
(b) Define the term:
(i) First Aid
(ii) First Aid Kit
5. (a) Which method would you use to separate each of the following mixture?
(i) Water mixed with kerosene
(ii) Iron powder mixed with sand
(iii) Ammonium chloride crystals mixed with sodium chloride crystal
(iv) Water mixed with alcohol
(b) Give one function of each of the following apparatus in the chemistry laboratory.
(i) Spatula .
(ii) Gas jar .
(iii) Lie big condenser ..
(iv) Motor and pestle
(v) Wire gauze ..
6. (a) Mention any three laboratory rules
(b) Name any three places or areas where the knowledge of Chemistry is applied.
7. (a) Mention any four instruments found in First Aid Kit
(b) Write the names of the following processes of changing matter from one state to another.
(i) Solid to liquid
(ii) Liquid to gas
(iii) Gas to liquid
(iv) Liquid to solid
8. (a) Define with one example:
(i) Element
(ii) Compound
(b) Compare physical change and chemical change.
9. (a) Why are there laboratory rules? (Give two reasons)
(b) Suppose your laboratory does not have any water, mention two possible dangers of using it.
10. (a) Differentiate hypothesis from analysis.
(b) Effective use of the four senses of observation is important before a chemist can make conclusion. With four points, show how the senses are used as tools of observation during experimentation by giving one example for each.
(i) .
(ii) .
(iii) . .
FORM ONE CHEMISTRY EXAM SERIES 1
FORM ONE CHEMISTRY EXAM SERIES 1
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







