PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM THREE
TERMINAL EXAMS MAY – 2023
032/1
Time: 3 Hours
Instructions
- This paper consists of section A, B and C with a total of thirteen (13) questions.
- Answer all questions in this paper
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used
Atomic masses:
H=1, C=12, O=16, N=14, Pb=108
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4 dm3
1 Faraday = 96,500 coulombs
Standard pressure = 760 mm Hg
Standard temperature = 273 K
1 litre = 1dm3 = 1000cm3
SECTION A
- Choose the correct answer from the alternatives given below
(i) The best chemical warning signs that should be put on bottles containing kerosene is ……….
- Corrosive
- Toxic
- Flammable
- Explosive
- Harmful
(vii) What volume of hydrogen gas will be produced when 1.3g of zinc granules react completely with excess dilute sulphuric acid at s.t.p?
- 223cm3
- 130cm3
- 220cm3
- 440cm3
- 448cm3
(x) The following reaction 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O (l) is an example of a
- Redox reaction
- Combination reaction
- Esterification
- Neutralization reaction
- Decomposition reaction.
(iv) 10cm3 of 0.4M Sodium Hydroxide are added to 40cm3of 0.2M hydrochloric acid. The resulting mixture will be
- Neutral
- Alkaline
- Dilute
- Acidic
- Amphoteric
(viii) A metal nitrate which will not give a metal oxide on heating is:
- Calcium nitrate
- Silver nitrate
- Lead nitrate
- Copper nitrate
- Zinc nitrate
(ix) When nitrogen gas is formed covalently how many electrons are shared between nitrogen atoms.
- 2
- 3
- 6
- 5
- 4
- Which of the following substances represent a group of acid oxides?
- Carbon dioxide, carbon monoxide and Sulphur dioxide.
- Sulphur trioxide, Nitrogen dioxide and Nitrogen monoxide
- Carbon dioxide, Sulphur dioxide and dinitrogen oxide
- Sulphur trioxide, carbon dioxide and Nitrogen dioxide
- Carbon monoxide, nitrogen oxide and sulphur dioxide.
- The reason why white anhydrous copper (II) Sulphate turns blue when exposed in Atmosphere is that it
- Absorbs water vapour
- Reacts with oxygen
- Reacts with carbon dioxide
- Become dry
- Release water to the Atmosphere
- Which action should be taken immediately after concentrated sulphuric acid is spilled on the skin?
- It should be rinsed off with large quantities of running water.
- It should be neutralized with concentrated NaOH
- The affected area should be wrapped tightly and shown to a medical health provider
- It should be Neutralize with solid CaCO3
- It should be neutralized with concentrated KOH
(x) The following is an example of organic acid
- Hydrochloric acid
- Phosphoric acid citric acid
- Citric acid
- Nitric acid
- Carbonic acid
- Match the item in LIST” A” with the correct response in LIST” B”
| LIST A | LIST B |
| (i) Methyl orange indicator (ii) Calcium hydroxide (iii) pH 2 (iv) Neutralization reaction (v) Sodium hydrogen sulphate |
|
SECTION B. (70 MARKS)
ANSWER ALL QUESTIONS FROM THIS SECTION
- (a) If 0.5g of hydrogen gas are exploded in air, What is the mass of water formed?
(b) 2FeCl3(aq) + H2S(g) → 2FeCl2(aq) + 2HCl + S(s)
From above equation, calculate the mass of iron (II) Chloride formed by the excess of hydrogen sulphide gas on a solution containing 54.0g of iron (III) chloride.
- (a)Find the molarity of a sample of sulphuric acid containing 98% by weight H2SO4 and having a density of 1.63gcm-3.
(b)Find the concentration in molarity of 2.70g of Sodium carbonate dissolved in 250cm3
- 70.0cm3 of X2CO3 solution required 50.0cm3 of 0.2M HCI for complete neutralization.
- Write the balanced chemical equation of the reaction.
- Calculate the concentration of X2CO3 in
- Molarity
- g/dm3
- If compound X2CO3 has molar mass equal to 106g, calculate the relative atomic mass of X.
- What is the name and symbol of elements X.
- Identify the period and group of element X.
- An electric current was passed in series through solutions of calcium chloride and copper (II) sulphate. Carbon electrodes were used in both electrolytes.
If 1.5 litres of chlorine measured at S.T.P were produced, what volume of oxygen would also be produced? What mass of copper was produced?
- Describe the industrial application of electrolysis.
- State whether the reaction:
will proceed forward or backward under these conditions:
- Pressure is increased,
- Temperature is lowered,
- More ammonia gas is removed,
- Hydrogen gas is reduced if other factors are maintained.
- (a)What is the source of the following in Haber process:
- Hydrogen
- Nitrogen
(b)What is the role of Silica gel in Haber process?
(c)Vanadium pentoxide is generally used as a catalyst in the contact process
Comment
(d) A catalyst can shift the position of a chemical equilibrium. Comment.
- The alternative energy sources for the future could be biogas, geothermal, nuclear, wave, tide, wind and hydroelectric comment.
SECTION C. 15 MARKS
ANSWER QUESTION 11.
- Enumerate four chief ores of iron.
- How is iron extracted from its ore?
- What is the role of the limestone in the extraction of iron?
FORM THREE CHEMISTRY EXAM SERIES 128
FORM THREE CHEMISTRY EXAM SERIES 128
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM THREE-2022
Time: 3 Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A ( 15 Marks)
Answer all questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- Chemistry is a study of
- The chemicals used in the laboratory
- An experiment carries out in industries.
- The composition , structure and properties of matter
- All scientific processes.
- Hassan wants to grid the granules of a certain chemical to fine powder. The apparatus he will use include a.
- Pestle and filter funnel
- Round-bottomed flask and trough
- Mortar and pestle.
- Bunsen burner and filter paper.
- Which of the following is a physical change?
- Milk left on the counter turns sour
- Common salt dissolves complete in water
- A forest fire burns all the trees
- Fruits are fermented to produce wine
- When a substance is heated and change from solid directly to a vapor the process is called
- Condensation
- Dissolving
- Sublimation
- Melting
- Which of the following gases if mixed with Hydrogen produce a very hot flame of up to 3000oC.
- Oxygen
- Neon
- Chlorine
- Argon.
- An isotope of Cadmium has an atomic number of 48 and mass number of 112. This mean that the Cadmium atom has.
- 48 protons, 64 neutrons, 48 electrons
- 64 protons, 48 neutrons, 64 electrons
- 48 protons, 112 neutrons, 48 electrons
- 112 protons, 112 neutrons, 112 electrons
- The reaction between Silver nitrate and Sodium chloride to form Silver chloride and Sodium nitrate is an example of a ………………. Reaction.
- Direct combination
- Simple displacement
- Double displacement
- Decomposition.
- Which of the following pairs of oxide are gaseous at room temperature?
- Carbon dioxide and copper (II) oxide
- Sulphur dioxide and copper (II) oxide
- Carbon dioxide and Sulphur dioxide
- Copper (II) oxide and Iron (II) oxide
- Ammonium chloride reacts with sodium hydroxide solution on warming. The net ionic equation for the reaction is.
- H+(aq) + OH-(aq) → H2O(l)
- NH+(aq) + OH-(aq) → NH3(aq) + H2O(aq)
- Na+(aq) + Cl-(aq) → NaCl(aq)
- 2NH4+(aq) + 2Cl-(aq) → NaCl(aq) + Cl(g) + H2(g)
- Which of the following is not a property of Hydrogen gas?
- It support combustion
- It is slightly soluble in water
- It is less denser than air
- It is colorless and odorless
2. The following are the matching items .Match the correct item in LIST B corresponding one from LIST A. Write the letter in answer sheet provided.
| LIST A | LIST B |
|
|
SECTION B ( 70 Marks)
Answer all questions in this section.
- a) i. What is an air?
ii. Is air a compound or mixture? Give four (4) reasons to support your answer.
b) State the methods of separating the following mixture. Give a reason to support your answer.
- Kerosene and water
- Muddy water
- Ethanol and milk.
- a). Define the following terms
- First Aid
- First aid kit.
b) Give five (5) items found in the First aid kit and their uses.
c) What First aid do you give to a person who has fainted?
- a) Differentiate between the following terms.
- Chemical reaction and chemical equation.
- Reactants and products
- Displacement reaction and Double displacement reaction.
b) Complete and balance the following reaction.

 + → Mg(NO3) (aq) + Zn(s)
+ → Mg(NO3) (aq) + Zn(s)
- Pb(NO3)2 (s) + Na2SO4 (aq) →
 Pb(NO3)2 (s) Heat
Pb(NO3)2 (s) Heat
- a) Explain the meaning of the following terms.
- Mole
- Molarity
b) i. State the Avogadro’s law.
ii. Mention two (2) applications of Avogadro law
- a) By using a well labeled diagram explain the preparation of oxygen using hydrogen peroxide.
b) List three chemical properties of oxygen.
- a) Name the following compounds according to the IUPAC system of nomenclature
- Fe2(SO4)3
- KMnO4
- H2SO4
b) What is fuel?
c) State four (4) the characteristics of good fuel.
- a) Define
- Atom
- Isotopes
b) i. What is an electronic configuration?
ii. Give three (3) applications of electronic configuration.
- a) Distinguish between the following terms
- Corrosive and irritant
- Radioactive and explosive
- Solution and suspension
b) i. What is flame?
ii. Differentiate between luminous and non-luminous flame. Give four (4) points.
- a) Explain the meaning of the following terms
- Empirical formula
- Molecular formula
- The compound of carbon and hydrogen has the empirical formula CH3. Find its molecular formula if its relative molecular mass is 30 (r.a.m carbon = 12).
- a) State four (4) natural sources of water.
b) Explain four (4) economic importance of water.
SECTION C ( 15 Marks)
Answer one (1) question from this section.
- a) Explain the meaning of each of the following terms
- Concentration of the substance
- Molar mass
- Stoichiometry
b) Calculate the number of moles in each of the following substances
- 1.064g of Magnesium nitrate
- 1.397g of Copper (II) sulphates
- 3.67dm3 of Sulphur dioxide at s.t.p
c) Calculate the number of ions present in 30g of Aluminum sulphates.
14. With the aid of a chemical equation, describe how you would prepare pure solid sodium chloride by the action of an acid and a base.
FORM THREE CHEMISTRY EXAM SERIES 83
FORM THREE CHEMISTRY EXAM SERIES 83
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY 1TERMINALEXAMINATION
FORM THREE-2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (20 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
- 1.4 g of potassium hydroxide is dissolved in water to form 250 cm3 of solution. What is the molarity of this solution?
- 0.01 M
- 0.1 M
- 1.4 M
- 5.6 M
- 6.0 M
- In the blast furnace carbon monoxide is prepared by passing carbon dioxide over a redhot coke. Carbon dioxide is
- an accelerator
- an oxidizing agent
- a reducing agent
- a catalyst
- oxidized.
- A catalyst can be described as a substance
- that alters the rate of reaction
- that slows down the rate of reaction
- used in every reaction so as to speed up rate of reaction
- that starts and speeds up the rate of reaction
- that terminates chemical reaction.
(iv) A covalent bond is formed when
- a metal combines with a nonmetal
- potassium and oxygen combine
- ammonia is formed
- two metals combine
- atom looses an electron.
(v) A solvent can be obtained from a solution by
- evaporation followed by decantation
- filtration and condensation
- evaporation and filtration
- evaporation and condensation
- crystallization followed by sublimation.
(vi) Aqueous sugar solution is a poor conductor of electricity because
- water and sugar are covalent compounds
- water is a nonelectrolyte
- sugar is a nonelectrolyte
- sugar is covalent when in liquid form
- sugar dissolves completely in water.
(vii) In order to produce the greatest amount of hydrogen in a short time, one gram of magnesium ribbon should react with
- 10 cm3 of 0.5 M sulphuric acid
- 40 cm3 of 0.5 M acetic acid solution
- 40 cm3 of 0.5 M sulphuric acid solution
- 20 cm3 of 1 M sulphuric acid solution
- 20 cm3 of 1 M acetic acid solution.
(viii) Fractional distillation process of a mixture of water and ethanol is possible because
- water and ethanol have the same boiling point
- water has lower boiling point than ethanol
- ethanol has lower boiling point than water
- water and ethanol form partially immiscible liquid solution
- water and ethanol are immiscible liquids.
(ix) Which of the following substances represent a group of acidic oxides?
- Carbon dioxide, carbon monoxide and sulphur dioxide
- Sulphur trioxide, nitrogen dioxide and nnitrogen monoxide
- Carbon dioxide, sulphur dioxide and dinitrogen oxide
- Sulphur trioxide, carbon dioxide and nitrogen dioxide
- Carbon monoxide, nitrogen oxide and sulphur dioxide.
(x) What will the molarity of a solution which contains 26.5 g of anhydrous sodium carbonate in 5 dm3 of solution?
- 0.05 M
- 0.25 M
- 5.30 M
- 0.025 M
- 0.50 M
2. Match the items in LIST A with the responses in LISTB by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. Hydrogen has can be prepared by passing steam over heated magnesium ribbon as shown in the figure 2.
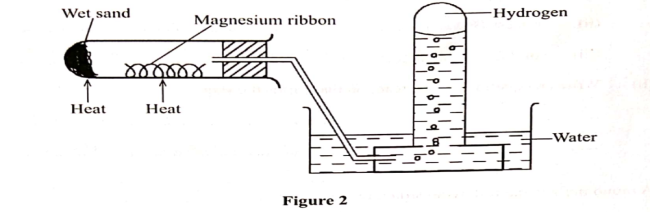
(a) Write an equation for the reaction that produces hydrogen gas.
(b) Explain why the delivery tube must be removed from beneath the water before heating is stopped.
(c) Explain why sodium metal is not suitable for this experiment.
4. A solution contains 40.3g of substance XOH per litre .250.0cm3of this solution required 30.0cm3 of 0.3M sulphuric(VI)acid for complete neutralisation.
(a) Calculate the number of moles of XOH that reacted.
(b) Determine the relative atomic mass of X.
5. (a) Explain the following observations:
(i) The colour of aqueous copper(ii) sulphate fades when a piece of magnesium metal is dropped into the solution.
(ii) A piece of iron bar is coated with a brown substance when left in the open on a rainy day.
6. A mass of 1.24g of a divalent metal was deposited when a current of 6A was passed through a solution of a metal sulphate for 12 minutes.Determine the relative atomic mass of the metal( Faraday = 96,500 C mol-1
(d) State two application s of electrolysis.
7. 30.0 cm3 of aqueous sodium hydroxide containing 8.0 g per litre of sodium hydroxide were completely neutralised by 0.294 g of a dibasic acid. Determine the relative formula mass of the dibasic acid. (Na = 23.0 ; O = 16.0 ; H 1.0)
8(a). Using iron filings, describe an experiment that can be conducted to show that oxygen is present in air.
(b) Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare? Explain. (3 marks)
9. (a) Name two ores of iron.
(b) Describe how the amount of iron in a sample of iron(III) oxide can be determined.
10.(a) Give three advantages of using chemical equations over word equations.
(b) You are provided with a compound composed of 22.2% zinc, 11.6% sulphur, 22.3% oxygen, and the rest percentage is water of crystallization. Calculate the molecular formula of the compound if its molecular mass is 283.
11.(a) (i) Name the compound which causes temporary hardness of water and the compound which causes permanent hardness of water.
(ii) Write one balanced chemical equation in each case to show how to remove temporary and permanent hardness of water.
(b) State four steps employed in the extraction of moderate reactive metals.
12.(a) Giving three reasons, explain why air is said to be a mixture of gases.
(b) (i) People suffering from heart burn usually use wood ashes for relief. Mention characteristic which makes the ashes to be used for heart burn relief.
(ii)Give four compounds found in laboratories which show the same characteristics as ashes.
SECTION C (15 Marks)
Answer one (1) question in this section.
13. Describe four common stages for the extraction of metals. Does the extraction of gold follow all four stages? Give reasons.
14. Read the following information carefully then answer questions that follow: 25 cm3 of potassium hydroxide were placed in a flask and a few drops of phenolphthalein indicator were added. Dilute hydrochloric acid was added until the indicator changed colour. It was found that 21 cm3 of acid were used.
(b) (i) What piece of apparatus should be used to measure out accurately 25 cm3 of sodium hydroxide solution?
(ii) What colour was the solution in the flask at the start of the titration?
(iii) What colour did it turn when the alkali had been neutralized?
(c) (i) Was the acid more concentrated or less concentrated than the alkali? Give reasons for your answer.
(ii) Name the salt formed in the neutralization.
(iii) Write an equation for the reaction.
(d) Utilizing the given information describe how you can obtain pure crystals of the salt.
FORM THREE CHEMISTRY EXAM SERIES 50
FORM THREE CHEMISTRY EXAM SERIES 50
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, LOCAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY- TERMINAL EXAMINATION-MAY
FORM THREE
TIME: 2HRS 2020
NAME:_______________________________________CLASS:___________
INSTRUCTIONS
- This paper consists of 3 sections A, B,and C
- Answer all questions in all sections
- Phones and electronic calculators are not allowed
- All work should be done using blue or black pen
- The following information and constants may be useful
H=1, N=14, O=16, C=12, Fe=56, Pb=207, Cl=35.5, Ca=40, Mn=55, K=39, 1litre=1dm3=1000cm3
Avogadro’s constant=6.02x1023 particles
1 faraday=96500 coulombs
GMV at STP=22.4dm3
SECTION A (20 MARKS)
1. For each item (i-x) choose the correct answer from given alternative and write it beside the item number in answer booklet provided.
(i) An element in periodic table with atom number 18 belongs to which of the following
- Group 1 and period 1
- Group O and period III
- Group III and period III
- Group V and period IV
- Group VII and period IV
(ii) The ionic equation when ammonium chloride react with sodium hydroxide is
- 2NH+4(aq) + 2Cl-
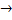 2NH3 + Cl2 + H
2NH3 + Cl2 + H - NH+4 + OH-
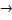 NH3 + H2O
NH3 + H2O - Na+ + Cl-
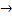 NaCl
NaCl - 2NH+4 + 2Cl-(aq)
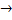 2NH3(g) + 2Hcl(g)
2NH3(g) + 2Hcl(g) - H+ + OH-
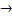 H2O
H2O
(iii) The reason why white anhydrous copperII sulphate turns Blue when exposed to atmosphere is
- Reacts with carbon dioxide
- Reacts with oxygen
- Becomes dry
- Absorbs water vapour
- Decomposes
(iv) Chemical change means
- Change in reversible
- Can easily be separated
- Change is complete
- Produces no change in mass
- New substance is produced
(v) If a stead current of 2 amperes was passed through an aqueous solution of ironII sulphate for 15 minutes, then, mass of iron deposited will be.
- 54g
- 56g
- 0.54g
- 28g
- 0.52g
(vi) Which of the following solutions is the most concentrated?
- 50g of calcium carbonate in 100cm3 of water
- 60g of sodium chloride in 200cm3 of water
- 65g of potassium nitrate in 100cm3 of water
- 120g of potassium suphate in 200cm3 of water
- 50g of sodium hydroxide in 200cm3 of water
(vii) Copper can be separated from mixture of zinc and copper by adding to the mixture
- Concentrated sulphuric acid
- Dilute sulphuric acid
- Aqueous solution of ZnSO4
- Catalyst
- Concentrated nitric acid
(viii) 10cm of 0.4M sodium hydroxide are added to 40cm3 of 0.2M Hcl. The resulting mixture will be
- Neutral
- Alkaline
- Dilute
- Acidic
- Amphoteric
(ix) The only metal which does not react with dilute Hcl is
- Magnesium
- Alluminium
- Copper
- Zinc
- Sodium
(x) During electrolysis of molten aluminum oxide; 3 faradays were needed to deposit one mole of aluminum. The number of electrons of aluminum will be:
- 6.02 X 1023
- 1.806 X 1023
- 18.06 X 1023
- 180.6 X 1023
- 1806 X 1023
2. Match the items in list A with responses in list B by writing the letter of correct response beside item number in separate answer sheet.
| LIST A | LIST B |
| (i) Its nitrate decomposes to metal, nitrogen dioxide and oxygen (ii) Its chloride is used as a drying agent (iii) Its carbonate is used to remove hardness of water (iv) It is stored paraffin (v) It hydride ion is metallic in nature (vi) Exists into two main physical forms (vii) Greenish- yellow gas (viii) Forms insoluble sulphate (ix) Reacts with carbon dioxide to form an oxide (x) Used as sacrificial element in catholic protection |
|
SECTION B (54 MARKS)
3. a) Why do chemistry laboratory exits open outward?
State uses of any four items in first Aid Kit
b) i) Arrange the following metals in order of increasing reactivity – zinc, magnesium, calcium, copper, mercury
ii) Which of the following metals b (i) reacts with steam forming an oxide which is white when cold and yellow when hot?
4. a)20cm of solution containing 7g dm3 sodium hydroxide were exactly neutralized by 25cm of 0.1M Hcl. Calculate the concentration of sodium hydroxide in moles per dm
b) Give two examples of
(i) Gaseous solution
(ii) Solid solution
5. a)The table below show part of periodic table study it and answer questions that follow
| H |
| He | |||||
| Li | Be | B |
|
|
| F |
|
|
|
| Al | Si | P | S | Cl | Ar |
Fill all missing elements
(i) Write the reaction equation involved in industrial manufacture of sulphuric acid in contact process; starting with sulphur metal
(ii) Explain why sulphur trioxide is not dissolved directly in water to obtain sulphuric acid
6. a)Explain how you can separate crystals of copper II sulphate from pieces of broken glasses
b) With aid of equation explain how washing soda removes hardness of water
7. Table below give information about composition of three samples of water Mineral content per Mg per litre
| Ions | Kahama | Maswa | Bukombe |
| Ca2+ | 28 | 82 | 18 |
| Mg2+ | 14 | 41 | 13 |
| Cl- | 53 | 7 | 22 |
| Na+ | 7 | 143 | 39 |
| HCO-3 | 281 | 5 | 93 |
| SO42- | 2 | 14 | 16 |
(i) State two ways in which these ions get into water
(ii) Give two reasons, state hardest water sample
(iii) State two ways that can be used to remove hardness in (II)
b) Write the following molecular equation to ionic
(i) Fe(s) + CUSO4(aq) → FeSO4(aq) + Cu(s)
(ii) Na2SO4(aq) + Bacl2(aq) → BaSO4(g) + 2Nacl(aq)
State the type of chemical reaction
(8) a) When a burning splint is introduced in gas for containing CO2, the flame goes off.
i) What two properties of CO2 does this experiment lustrate?
ii) What type of equipment widely used in everyday life makes use of these two properties?
b) The equation below show dissociation of calcium carbonate
CaCO3(s) → CaO(s) + CO2(g) H
(i) Is the forward reaction endothermic or exothermic?
(ii) What factors favour forward reaction?
(iii) What will be the effect on proportion of CaCO3 in the equilibrium mixture if temperature is decreased?
9. a)Lead nitrate decompose on heating as follows
2 Pb (NO3)2(g) → 2PbO(S) + 4NO2 (g) + O2(g)
112dm3 of O2 were collected at STP when a sample of a lead nitrate was completely decomposed on heating. Calculate mass of load II nitrate in the sample.
b) Define the following terms
(i) Mole
(ii) Titrant
(iii) Analyte
(iv) Molar solution
10. a)Differentiate between
(i) A base and alkali
(ii) Atom and isotope
b) An organic compound P consists of 52.2% carbon, 13% hydrogen, and 34.8% oxygen.
The vapour density of P is 23. Calculate the molecular formula of compound P
11. Describe the extraction of iron using the blast furnace
SECTION C (26 MARKS)
12. Assume that you are a chemist in a chemical plant and want to produce 100litres of chlorine gas per hour so as to reach company goal of producing 2400litres every day. What current of electricity will you allow to flow per hour?
13. A solution of hydrated sodium carbonate was nitrated with 1.68M nitric acid solution. 30cm3 of the solution required 28.75cm3 of nitric acid for complete reaction. If the solution was prepared by dissolving 12.056g of the carbonate to make 600cm3 of solution, determine the molecules of water of crystallization in hydrated sodium carbonate.
END
FORM THREE CHEMISTRY EXAM SERIES 13
FORM THREE CHEMISTRY EXAM SERIES 13
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







