THE PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
CHEMISTRY FORM ONE TERMINAL EXAMINATION
CODE 032
TIME: 2:30 HOURS
INSTRUCTIONS.
- This paper consists of section A, B and C with the total number of ten(10) questions
- Answer all questions in each section
- Section A carries (15) marks, section B (70) marks and section C carries (15) marks
- All writing must be in blue/black ink except drawing which must be in pencil
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A (15 Marks)
Answer all questions in this section
1. For each of the items (i) – (x) choose the correct answer from among the given alternatives and write its letter beside the item number in the answer sheet provided
- A new substance is formed when baking soda and vinegar are mixed. This is evidence of:
A. A physical change
B. A chemical change
C. A temperature change
D. A change in size
- Which product heavily relies on the work of chemists in its creation?
A. Fertilizers for growing crops
B. Fabrics for durable clothing
C. Concrete for building structures
D. All of the above
- To design a water filtration system for a community, an engineer needs knowledge of chemistry to:
A. Determine the best materials for capturing impurities.
B. Understand how different substances interact.
C. Predict the outcomes of different filtering methods.
D. All of the above
- During an experiment, you notice an unexpected color change. To follow good scientific practice, you should:
A. Record the change and try to explain why it occurred
B. Ignore it and continue as planned
C. Stop the experiment immediately
D. Tell your friend but don't write it down.
- Wearing safety goggles in the lab is essential because they:
A. Protect your eyes from potential chemical splashes.
B. Make you look like a scientist.
C. Prevent you from touching your face.
D. Help you see the experiment more clearly.
- You're unsure how to dispose of a chemical after an experiment. What should you do?
A. Pour it down the sink
B. Mix it with another chemical to see what happens.
C. Leave it at your workstation for the teacher.
D. Ask your teacher for specific disposal instructions.
- Your friend accidentally gets a small amount of a chemical on their hand. They should:
A. Quickly go to the chemical wash station.
B. Wipe it off on their clothes.
C. Tell a teacher and wait for directions.
D. Try to ignore the feeling.
- You need to heat a liquid as part of your experiment. Which apparatus is the safest and most appropriate?
A. Beaker and Bunsen burner
B. Graduated cylinder and candle
C. Erlenmeyer flask and hot plate
D. Test tube and lighter
- A triangular sign with a flame symbol means the substance presents a:
A. Electrical hazard
B. Biohazard
C. Fire hazard
D. Environmental hazard
- Seeing a test tube with cracks on a chemical bottle likely indicates:
A. The chemical is very old.
B. The chemical may be heat sensitive.
C. The chemical could be damaged or unsafe.
D. The chemical is perfectly safe to use.
2. Match the laboratory apparatus in Column A to its correct use in Column B.
| Column A | Column B |
| A. Heating liquids or solids B. Holding and mixing liquids C. Storing liquids for long periods of time (with a stopper) D. Measuring precise volumes of liquids E. Performing small-scale reactions
|
SECTION B: 70 MARKS
3. The following are laboratory apparatus used in Chemistry. Name them and give their uses.
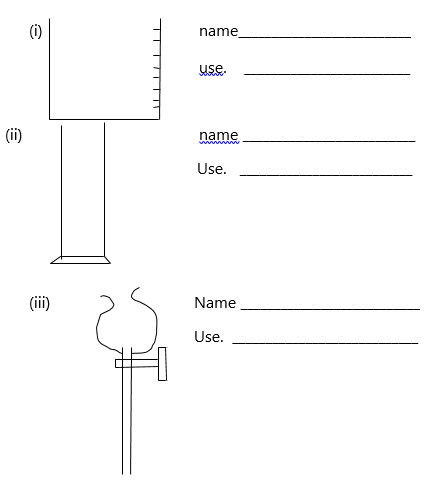
(b). Give two reasons why most laboratory apparatus are made of glass.
4.(a) Name three apparatus that can be used to measure accurate volume
(b) Classify the following as chemical change or physical change;
- Burning of candle
- Burning of paper
- Rusting
- Evaporation of water
- Sublimation of iodine
(c) Name four kinds of materials that can be used to make laboratory apparatus
5. (a) Define the term chemistry
(b) Name areas where knowledge of chemistry finds applications.
(c) Name four home made products which are made using the knowledge of chemistry
6.(a) What is a chemistry laboratory?
(b) Explain why a laboratory is different from other buildings
(c) Mention accidents that can occur in laboratory
7.(a) Define first aid
(b) Why is first Aid important to an accident victim?
© Name four items that can be used when giving first aid to an accident victim
8. (a) Define the term matter
(b) Name three states of matter
(c) Explain why it is possible to separate of mixture of sodium chloride and Iodine
9. (a) Define the following terms
- An atom
- Molecule
- Element
(b) Differentiate between a chemical change and a physical change
SECTION C: 15 Marks
10.(a) Complete the following table;
| Element | Symbol | Element | Symbol |
| Sodium |
|
| Hg |
|
| K | Copper |
|
| Sulphur |
|
| C |
| Iron |
| Hydrogen |
|
(b) Discuss the importance of studying chemistry.
FORM ONE CHEMISTRY EXAM SERIES 171
FORM ONE CHEMISTRY EXAM SERIES 171
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM ONE
TERMINAL EXAMS-MAY– 2023
033
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carry seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
1. For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
(i) Which of the following is the correct sequence of the last two steps you should follow during the scientific procedure?
- Hypothesis formulation and conclusion
- Observation and problem identification
- Experimentation and conclusion
- Problem identification and hypothesis formulation
- Interpretation of data and conclusion.
(ii) Which of the following is not a component of the First Aid Kit?
- Goggles
- A pair of scissors
- Dropper
- Gloves
- Razor blade
(iii) Which one of the following processes is a chemical change?
- Butter melts on warm toast
- Water evaporates from the surface
- Juice in a bottle freezes
- Food scrap turns into compost
- Wet cloth dries
(iv) When you melt a piece of iron, it undergoes:
- Sublimation
- Physical change
- Chemical change
- Combination
- Synthesis
(v) The most abundant element on the earth is:
- carbon
- iron
- nitrogen
- Oxygen
- Sulphur
(vi) Petrol is an example of:
- corrosive substance
- flammable substance
- irritating substance
- toxic substance
- Oxidant
(vii) The boiling point of pure water at sea level is 1000C and that of ethanol is 780C. The mixture of ethanol and water can be separated by:
- Filtration process
- Fractional distillation process
- Layer separation process
- Sublimation process
- Evaporation
(viii) When a chemist studies a substance he/she is interested in its:
- Force of attraction
- Properties
- Shape
- Smell
- Density
(ix) In the Bunsen burner a sooty flame is most likely to be formed when the:
- Air holes are fully closed
- Air holes are opened
- Flame is noisy
- Flame is smaller and hotter
- Methane gas is used
(x) The process by which water is converted into water vapour or steam is called:
- Condensation
- Evaporation
- Precipitation
- Transpiration
- Fusion
2. Match the terms in LIST B with the correct description in LIST A and write the letter of correct answer in spaces provided.
| LIST A | LIST B |
|
|
SECTION B. 70 MARKS
ANSWER ALL QUESTIONS FROM THIS SECTION.
3. (a) Define the following terms:
(i) Brownian motion
(ii) Compound
(b) Identify whether the following is a physical or chemical change:
(i) Cutting aluminium foil into pieces
(ii) Lighting a match.
(c) How can you separate the following mixtures? Briefly explain.
(i)Water and kerosene
(ii)Salt and water
(iii)Ethanol and water
4. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
5. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv. Sterile gauze
6. (a) Differentiate the following terms:
(i) An element and an atom
(ii) A compound and a mixture
(b) Melting of ice and burning of papers are two processes that happen almost daily in our life.
(i) Identify the two actions as being physical or chemical change.
- Ice melting __________________________________________________
- Paper burning ________________________________________________
(ii) Mention two significant things that happen when the paper is burnt:
(c) What are the names of elements represented by the following symbols?
(i) K _________________________________________________________
(ii) S _________________________________________________________
(d) Why some elements have their symbols written in one letter while others have two letters? (e.g. N and Na)
7. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
8. Describe the eight stages of lighting a Bunsen Burner
9.(a) Explain each of the following terms;
- Burn
- Bruises
- Fainting.
(b) Write the differences between metals and Non-Metals
10.
(a) Give the use of each of the following components which are found in the First Aid kit:
(i) Plaster .........
(ii) A pair of scissors
(iii) Cotton wool .........
(iv) Gloves .......
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
FORM ONE CHEMISTRY EXAM SERIES 129
FORM ONE CHEMISTRY EXAM SERIES 129
PRESIDENT OFFICE REGIONAL ADMNISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
CHEMISTRY FORM ONE
TERMINAL EXAMS-MAY– 2023
033
Time: 2:30 Hours
INSTRUCTIONS
- This paper consists of sections A, B and C with a total of ten (10) questions.
- Answer all questions
- Section A and C carry fifteen (15) marks each and Section B carry seventy (70) marks.
- All writing must be in blue or black ink except drawing which must be in pencil.
- Cellular phones and any unauthorized materials are not allowed in the assessment room.
SECTION A
1. For each of items (i) – (x) choose the correct answer from among the given alternatives and write its letter in box provided.
(i) Which of the following is the correct sequence of the last two steps you should follow during the scientific procedure?
- Hypothesis formulation and conclusion
- Observation and problem identification
- Experimentation and conclusion
- Problem identification and hypothesis formulation
- Interpretation of data and conclusion.
(ii) Which of the following is not a component of the First Aid Kit?
- Goggles
- A pair of scissors
- Dropper
- Gloves
- Razor blade
(iii) Which one of the following processes is a chemical change?
- Butter melts on warm toast
- Water evaporates from the surface
- Juice in a bottle freezes
- Food scrap turns into compost
- Wet cloth dries
(iv) When you melt a piece of iron, it undergoes:
- Sublimation
- Physical change
- Chemical change
- Combination
- Synthesis
(v) The most abundant element on the earth is:
- carbon
- iron
- nitrogen
- Oxygen
- Sulphur
(vi) Petrol is an example of:
- corrosive substance
- flammable substance
- irritating substance
- toxic substance
- Oxidant
(vii) The boiling point of pure water at sea level is 1000C and that of ethanol is 780C. The mixture of ethanol and water can be separated by:
- Filtration process
- Fractional distillation process
- Layer separation process
- Sublimation process
- Evaporation
(viii) When a chemist studies a substance he/she is interested in its:
- Force of attraction
- Properties
- Shape
- Smell
- Density
(ix) In the Bunsen burner a sooty flame is most likely to be formed when the:
- Air holes are fully closed
- Air holes are opened
- Flame is noisy
- Flame is smaller and hotter
- Methane gas is used
(x) The process by which water is converted into water vapour or steam is called:
- Condensation
- Evaporation
- Precipitation
- Transpiration
- Fusion
2. Match the terms in LIST B with the correct description in LIST A and write the letter of correct answer in spaces provided.
| LIST A | LIST B |
|
|
SECTION B. 70 MARKS
ANSWER ALL QUESTIONS FROM THIS SECTION.
3. (a) Define the following terms:
(i) Brownian motion
(ii) Compound
(b) Identify whether the following is a physical or chemical change:
(i) Cutting aluminium foil into pieces
(ii) Lighting a match.
(c) How can you separate the following mixtures? Briefly explain.
(i)Water and kerosene
(ii)Salt and water
(iii)Ethanol and water
4. a) During the rains many rivers fill with muddy water, which people use for drinking. This water has caused them to suffer from water borne diseases such as typhoid and diarrhea. Explain two simple methods that can be used to obtain clean water from these rivers muddy water.
b) One form one student from a certain school was given a task by her teacher to write the chemical symbols of the following elements; sodium, iron and chlorine. But the student did as shown below in 6b ( i, ii, and iii). Help him by identifying his mistakes and then write a correct symbol in each
i. Symbol of sodium is (So)
ii. Symbol of iron is (Ir)
iii. Symbol of chlorine is (CL)
5. (a) Do your think there is any reasons for providing first Aid to the Victim? If yes give four reasons.
(b)State the uses of the following items in first aid Kit.
i. The pair of scissors
ii. The whistle
iii. Petroleum Jelly
iv. Sterile gauze
6. (a) Differentiate the following terms:
(i) An element and an atom
(ii) A compound and a mixture
(b) Melting of ice and burning of papers are two processes that happen almost daily in our life.
(i) Identify the two actions as being physical or chemical change.
- Ice melting __________________________________________________
- Paper burning ________________________________________________
(ii) Mention two significant things that happen when the paper is burnt:
(c) What are the names of elements represented by the following symbols?
(i) K _________________________________________________________
(ii) S _________________________________________________________
(d) Why some elements have their symbols written in one letter while others have two letters? (e.g. N and Na)
7. (a) Most of laboratory apparatuses are made up of glass materials. Why? (Give three reasons)
(b) Briefly explain how to handle chemicals having the following warning signs.
(i) Flammable
(ii) Corrosive
(c) Why luminous flame produces soot?
8. Describe the eight stages of lighting a Bunsen Burner
9.(a) Explain each of the following terms;
- Burn
- Bruises
- Fainting.
(b) Write the differences between metals and Non-Metals
10.
(a) Give the use of each of the following components which are found in the First Aid kit:
(i) Plaster .........
(ii) A pair of scissors
(iii) Cotton wool .........
(iv) Gloves .......
(b) List four properties of each of the following:
(i) A luminous flame
(ii) A non-luminous flame
FORM ONE CHEMISTRY EXAM SERIES 128
FORM ONE CHEMISTRY EXAM SERIES 128
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY TERMINAL EXAMINATION
FORM ONE-2022
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- Chemistry helps us to explain
- Why and how matter undergoes changes
- What medicine to use when are fall sick
- How chemicals are used in agriculture
- How pesticides help in crop population
- A simple proof that chemical reactions takes place in our bodies is that
- We occasionally fall sick
- Doctors tell us in the hospital
- The food we eat or drinks we take are quite different from the waste products from our bodies
- We eat a balance diet.
- Which of the following is not a list of products of application of chemistry?
- Tables, chairs and bricks
- Shoes polish, antiseptics and toothpaste
- Soap, medicinal and perfume
- Fertilizer, pesticides and herbicides
- Chemistry is a scientific activity because
- Chemistry is studies in schools
- It’s an interesting subject
- Knowledge of chemistry is acquired through observation
- Knowledge of chemistry is acquired through experiment and logical reasoning
- A condition in which the lungs are unable to get enough oxygen is known as
- Shock
- Fainting
- Suffocation
- Choking
- Choking can be treated by
- Cardio pulmonary resuscitation
- Expose the victim to the air
- Perform Heimlich manoeuvie
- Put a victim in the recovery position
- If the poison is on the skin then
- Do not apply any ointment
- Move the person to where there is a fresh air
- Give the victim fluids or juice
- Give on oral rehydration drinks
- A careless student secretly tied to mix up different chemicals just out of curiosity. The most thing that could happen to the student was to
- Damage the school properly
- Be expelled from school
- Annoy the teacher
- Produced poisonous cases that could lead to death
- Which of the following is not true about First Aid?
- First aid avoids an already bad situation from worsening
- First and helps to reduce pain, brings hope and encouragement
- Every student should be trained on how to administer first aid
- It is given to an accidental victim before seeking the doctor’s advice
- What is the use of splints first aid?
- Used for cutting bandages or pieces of cloth
- Used for holding pieces of bandages or cloth together
- Used to support broken bones and is tied using bandages
- Used for picking up and holding things
2. Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
(b) Answer the given questions by writing the correct answer in the blank spaces provided.
(i) In which stage is the hypothesis tested during scientific investigation? . . . . . . . . . . . . . . . . .
(ii) On which factor does the physical state of a molecule depend? . . . . . . . . . . . . . . . . .
(iii) Which properties depend on the proportions of mixing substances in a mixture? . . . . . . . . . . . . . . . . .
(iv) What are the building blocks of matter? . . . . . . . . . . . . . . . . .
SECTION B: 56 Marks
Answer all questions
3. Fill in the blank space
- What is a flame? ________________________________________________________________________________________________________________________________________________________________________________________
- What is a heat? ________________________________________________________________________________________________________________________________________________________________________________________
- Name the type of the flame that is produced when the air hole of the Bunsen burner are half closed __________________________________________________________________
- What causes the striking back of a flame in the Bunsen burner.
Mention only three causes
- __________________________________________________________________________
- __________________________________________________________________________
- __________________________________________________________________________
- Mention two ways which striking back is recovered
- _____________________________________________________________________________
- _____________________________________________________________________________
4. Study the figure below and answer the question that follows:
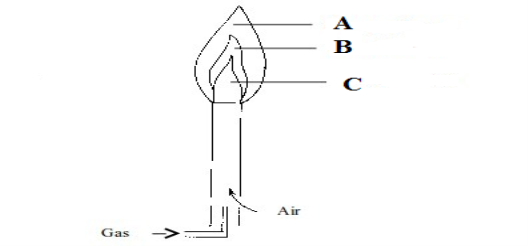
- Name the type of the flame indicate by the figure below _______________________________________________________
- Name the parts labeled: A _________________________________ B _________________________ C________________________________________
- What would happen if the march stick was placed in the part labeled C ____________________________________________________________________________________________
5. (a) List down ten laboratory rules
(b) With the help of diagram explain how each of the following lab apparatus works
(i) Condenser (ii) Burette (iii) pipette (iv) deflagrating (v) thermometer
(c) State two aims of the alchemists
(i) _____________________________________________________________________________
(ii) _____________________________________________________________________________
- What is the use of the following in the lab?
- Dark room _______________________________________________________________________
- Large windows __________________________________________________________________
- Circuit breaker __________________________________________________________________
6. Explain how chemistry is applied in the following field?
i)Communication ________________________________________________________________________________________________________________________________________________________________________________
ii)Transportation ________________________________________________________________________________________________________________________________________________________________________________
b)List down three common accidents that to occur in the chemistry lab and their possible causes
- ________________________________________________________________________________________________________________________________________________________________________________
- ________________________________________________________________________________________________________________________________________________________________________________
- ________________________________________________________________________________________________________________________________________________________________________________
c)Explain how you would administered first aid to a person having an electric shock at least four steps ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
7. With the help of a well labeled diagram of the Bunsen burner describe how the Bunsen burner work (the process involving lighting of the Bunsen burner)
8. With the help of a well labeled diagrams differentiate between the luminous flame and non luminous flame
9. With the aid of the diagram explain any four chemical signs that are found in the chemistry lab. Suggest their precaution measures.
FORM ONE CHEMISTRY EXAM SERIES 91
FORM ONE CHEMISTRY EXAM SERIES 91
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRYTERMINAL EXAMINATION
FORM ONE-2021
Time: 2:30Hours
Instructions.
- This paper consists of section A, and B with a total of 10 questions
- Answer all questions in spaces provided.
- Section A carries 20 marks, section B 80 marks.
- All answers should be written in the spaces provided.
- All communication devices, calculators and any unauthorized material are not allowed in examination room.
- Write your number on every page of your answer booklet.
- The following atomic masses may be used: H=1, N=14, O=16, S=32
SECTION A (20 Marks)
Answer All questions in this section.
- For each of the items (i)-(x), choose the correct answer from the alternatives given.
- The following skills acquired through studying chemistry except;
- Careful observation
- Accurate reporting
- Testing hypothesis experimentally
- Map reading skills
- Chemistry is a scientific activity because;
- Chemistry is studied in schools
- Chemistry is interesting subject studied through data collection and analysis.
- Knowledge of chemistry is acquired through observation, experimentation and logical reasoning.
- It involves study of living and non living things.
- The following are laboratory rules except;
- Turn off water and gas tap after use
- Test any unlabelled substance before using it in the laboratory.
- Put off flames which are not in use
- Remove obstacles from gang ways.
- Splints are among of the first aid kit equipments which of the following sentences set describes its use;
- Used to pick up and holding things
- Used for cutting bandages or pieces of clothes
- Used to support broken bones and is tied using bandages.
- Used for holding pieces of bandages or cloth together.
- The Bunsen burner is likely to contain soot when;
- The air hole is closed
- The air hole is open but not fully
- The air holes of Bunsen burner are completely open.
- The burner is not raised
- The gas supply is high
- Which of the following takes place in experimental stage?
- Rejection of hypothesis
- Acceptance of hypothesis
- Concluding the scientific findings
- Carry out investigations
- In scientific procedure, what would be the next step to follow after experiments?
- Data interpretation
- Conclusion
- Observation
- Investigation
- In her first experiment, Mwajuma dissolved sulphuric acid in water and heat was evolved. In her second experiment he dissolved sugar in water and no heat was evolved or absorbed she conducted that;
- Her first experiment was physical change
- Both of her experiments were physical change
- Her second experiment was chemical change
- Her first experiment was chemical change
- In most general sense the word combustion means …………….
- Burning substance in oxygen or air.
- Burning compound of carbon in air.
- Burning any fuel in air or oxygen
- By chemical combination accompanied by light and heat in which or more reactants is gaseous
- 10 grams of copper powder were placed initiation tube containing air. The tube was very strongly for half an hour. At the end experiment;
- Copper increased in mass in changed colour
- Copper melted into liquid copper form.
- The total mass of tube and its content decreased.
- Water vapour appeared in the tube.
2. (a) Match the items in List A with their correspondence items in List B.
| LIST A | LIST B |
|
|
(b) Fill the following gaps with the correct answer
- Mixture of solute and solvent…………………………………………
- A box for keeping items to be used for first Aid…………………………….
- Solid obtained after filtration……………………………………..
- An apparatus used to measure fixed volume of a liquid………………..
- The smallest particle of matter…………………………………..
- (a) Define the following terms;
- Combustion ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
- Combustible material ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
(b) Label the parts marked A, B, C, D, E and F in the diagram below;
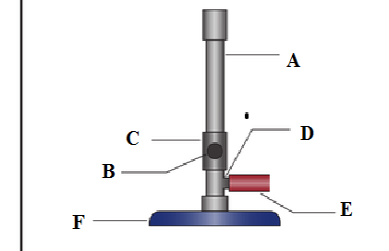
- ………………………………………..
- ………………………………………..
- ……………………………………….
- ………………………………………..
- ………………………………………..
- ………………………………………..
(c) What is the importance of parts labelled?
B ………………………………………………………………………………………
C ………………………………………………………………………………………
D ……………………………………………………………………………………….
- Fill the suitable word in the space provided;
- Bronze is an alloy formed by mixing ……………… and ……………………….
- Steel is an alloy formed by mixing ………………… and ……………………….
- …………………… is a solution in which like solvent used is not water.
- …………………….. is used to stir and mix substance uniformly.
- (a) Define the following terms and give one example in each case;
- A chemical symbol ……………………………………………………………………………………………………………………………………………………………………………………
- An element ……………………………………………………………………………………………………………………………………………………………………………………
- A compound ……………………………………………………………………………………………………………………………………………………………………………………
- Suspension ……………………………………………………………………………………………………………………………………………………………………………………
(b) Write names of elements represented by the following symbols
i. Au ……………………………………
ii. Ag …………………………………….
iii. Hg ……………………………………..
iv. P ………………………………………..
v. Sn …………………………………………
- Draw apparatus which are used to perform the following process in the laboratory;
| Process | Apparatus diagram |
| |
| (b)A flat apparatus at the base used to hold volume of liquid during experiment. | |
| © Wire placed on top of tripod stand. | |
| (d)Used for scooping small quantities of powder or crystalline chemicals. | |
| (e)Used for holding heating and mixing liquid – it measures estimate volume of liquids. |
- (a) Mention and explain briefly any five warning signs found in chemical containers
giving one example in each case.
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
(b) Distinguish between the following;
i. Hypothesis against fact ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii. Deposition against sublimation ………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(c) Point out any 6 differences between metals and non – metals ;
| Metals | Non metals |
- (a) Briefly explain any five methods of preventing rusting.
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
- ………………………………………………………………………………………………………………………………………………………………………………
(b) A certain pink coloured compound is heated to form blue colour. When water is
added to sample , it changed back to pink.
i. What change is demonstrated by the compound …………………………………....
ii. Explain the reasons for your choice.
…………………………………………………………………………………………………………………………………………………………………………………………...….
(c) State the type of changes demonstrated by the following phenomenon;
- Burning of firewood ……………………………………………………….
- Rotting of meat …………………………………………………………….
- Heating frying pan …………………………………………………………..
- Melting of butterfat …………………………………………………………
- Change of cloud to rain ……………………………………………………..
- Heating of iron rod …………………………………………………………..
- Your provided with the diagrams below, study it carefully and answer questions that follows;
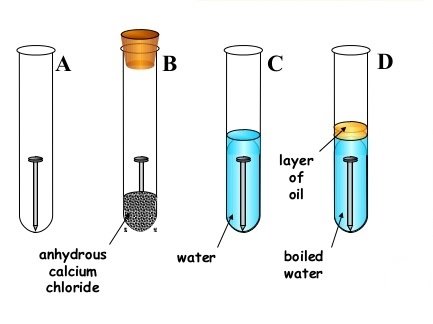
- State short and clear description about what will be observed in each test tube A – D after 2 or 3 days;
- Test tube A. ………………………………………………….
- Test tube B …………………………………………………..
- Test tube C. …………………………………………………..
- Test tube D. …………………………………………………..
- Why the water in the test tube D boiled the covered with oil. ……………………………………………………………………………………………………………………………………………………………………………………
- What is the function of calcium chloride in test tube B? …………………………………………………………………………………………………………………………………………………………………………………….
- What conclusion can you draw from your experiment? …………………………………………………………………………………………
- (i) Give two similarities between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Give two differences between combustion and rusting …………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
FORM ONE CHEMISTRY EXAM SERIES 55
FORM ONE CHEMISTRY EXAM SERIES 55
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







