PRESENT’S OFFICE, REGIONAL ADMINISTRATION
AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY FORM THREE
MID-TERM EXAMS – AUGUST – 2023
TIME: 2:30 HRS
INSTRUCTIONS
SECTION A
- For each of the items (i) – (x) choose the correct answer from among the alternatives given
- A beaker containing solid carbon dioxide in placed in a fume chamber at room temperature. The carbon dioxide became gaseous. Which process describes this change of state?
- Boiling
- Evaporation
- Condensation
- Sublimation
- Which of the chemical reactions release energy in form of light and heat?
- Combustion
- Decomposition
- Displacement
- Neutralization
- Precipitation
- Laboratory Technician prepared a solution containing 26.5g of anhydrous carbonate is 5dm3 of solution and provided to form four students to calculate its morality. Which among the following will be the possible answer?
- 0.05
- 0.25
- 1.25
- 5.3
- 0.025
- In a blast furnace carbon monoxide is prepared by passing carbon dioxide over a red hot coke. What is chemical role of carbon dioxide?
- An accelerator
- An Oxidizing agent
- A reducing agent
- A catalyst
- Oxidized
- What volume of hydrogen gas will be produced when 1.3g of Zinc granules react completely with excess dil.H2SO4 at STP?
- 223cm3
- 130cm3
- 220cm3
- 440cm3
- 448cm3
- 10cm3 of 0.4M sodium Hydroxide are added to 40cm3 of 0.2MHcl. The resulting mixture will be
- Neutral
- Alkaline
- Dilute
- Acidic
- Amphoteric
- A metal nitrate which will not give a metal oxide on heating is
- Calcium nitrate
- Silver nitrate
- Lead nitrate
- Copper Nitrate
- Zinc Nitrate
- Which substance can be reduced when heated with carbon
- Aluminum
- Calcium Carbonate
- Iron II Oxide
- Magnesium Oxide
- Sodium Oxide
- What numbers of Faradays of Electricity is required to deposit 4g of calcium from molten calcium chloride?
- 0.1
- 0.2
- 0.4
- 0.3
- 0.7
- The reaction between hydrogen and iodine is represented by H2(g) + I2(g)
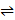 2HI(g)∆H= -xKj/Mol: The reaction is
2HI(g)∆H= -xKj/Mol: The reaction is
- Endothermic reaction
- Neutralization reaction
- Displacement reaction
- Decomposition reaction
- Match item in LIST A with correct response from LIST B
| LIST A | LIST B |
|
|
SECTION B (54 Marks)
Answer all questions
- (a)iron is extracted from various Ores by reduction in the blast furnace
- What is the Chief ore from which iron is extracted?
- Write equation for the reductions of the ore in blast furnace
- Explain the role of the following substances in the blast furnace, Limestone, coke
(b) If 0.5g of hydrogen gas in exposed to air. What mass of water will be formed?
- Juma drew a periodic table and then put a shadow on the element with atomic number 8
- What type of chemical bond is found between atoms of element
- Compound X contains 24.24% carbon, 4.04% Hydrogen and 71.72% Chlorine. Given that, the vapour density of X is 49.5
- Calculate molecular formula of compound X
- Draw and Name the Open structural formula of compound X
- (a)Hydrogen gas can be prepared by passing steam over heated magnesium ribbon as shown below
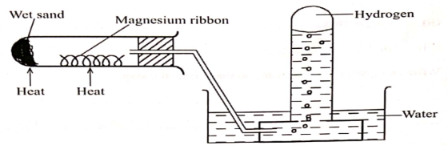
- Write equation for reaction that produce hydrogen gas
- Explain why the delivery tube must be removed from beneath the water before heating is stopped
- Explain why sodium metal is not suitable for this experiment
(b)Explain the following Observation
- The colour of aqueous Copper. II Sulphate fades when a place of Magnesium metal is dropped into the solution
- A piece of iron bar is coated with a brown substance when left in open on a rainy day
- (a) 30.0cm3 of aqueous sodium hydroxide containing 8.0g per litre of sodium hydroxide were completely neutralized by 0.294g of a dibasic acid. Determine the relative formula mass of dibasic acid (Na=23.0, O= 16.0, H=1.0
(b)Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare. Explain
- Figure below shows part of periodic table. The letters do not represent actual symbols of elements
| G |
|
| |||||
|
|
|
|
| I |
|
| V |
| K | L | M |
|
|
|
|
|
| J |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)(i)Select element which belong to the same chemical family
(ii) Write the formula of ions for elements in the same period
(b)The first ionization energies of two elements K and M at random are 577 Kjlmol and 494KJlmol
- Write the formula of the compound formed when L and I react
- Give one use of element V
- State another group that G can be placed in figure above
- How do reactivity of element J and K compare
- Distinguish between Temporary Hardness and Permanent Hardness of water basing on their ions
- Using equation show how each hardness can be removed
- Giving four reasons explain why people who use hard water can expect high cost than people using soft water.
- (a) Give good reason for the following
- Natural gas is popular in heating and cooking in homes
- Nuclear energy is not sustainable source of Energy
(b)State the main raw materials and process involved in manufacture of each of the following products
- Wood charcoal
- Coke
- Lamp Black
- Animal charcoal
- (a)Explain the meaning of each of the following terms
- Reversible reaction
- Dynamic equilibrium
(b)The industrial Oxidation of sulphur dioxide is summarized in equation below
2SO3(g) + O2(g) ![]() 2SO3(g) ∆H=94.9 KJlmol
2SO3(g) ∆H=94.9 KJlmol
What will be the effect of each of the trioxide?
- Increase in moles of Sulphur dioxide
- Increase in Temperature
- Decrease of moles of Sulphur dioxide
(c)Give one good reason for the following
- Fruits ripe faster during summer than during winter
- Steel wire get rust faster than iron nails
- (a)State faradays laws of Electrolysis
(b)Dilute silver nitrate solution was decomposed by the passage of electric current through it. What mass of Silver and what volume of Oxygen measured at Stp would be liberated in electricity?
FORM THREE CHEMISTRY EXAM SERIES 140
FORM THREE CHEMISTRY EXAM SERIES 140
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY-SEPT 2022
FORM THREE
032
Time:3 Hours SEPT, 2022
Instructions
- This paper consists of sections A, B and C with a total of fourteen (14)
questions.
- Answer all questions in sections A and B and one (1)question from section C.
- Sections A and C carry fifteen (15) marks each and section B carries seventy (70) marks.
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your name on every page of your answer sheets.
- The following constants may be used.
Atomic masses: H = 1, O = 16, C = 12, N = 14, Na = 23, Cl = 35.5, K = 39
Ca = 40
Avogadro’s number:![]()
GMV at s.t.p =![]()
1 Faraday = 96,500 coulombs.
Standard pressure = 760 mm Hg.
Standard temperature = 273 K.
1 litre =![]() =
=![]()
SECTION A (15 Marks)
Answerall questions in this section.
- For each of the items (i) – (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer sheet.
- A Bunsen burner is a chief source of heat in the laboratory that produces both blue and yellow flames. Which one among the following heat sources produce a blue flame:
A: spirit lampB:gas stoveC:kerosene stove
D:candleE:hurricane lamp
- “Water is referred to as the universal solvent.What does this statement mean?
A: it is commonly known liquid
B:it exists in all three states of matter
C:it dissolves more substances than any other known liquid.
D:it is used for cooking, drinking and washing bodies and clothes
E:it is colourless, odourless and tasteless liquid
- Kiraka was suffering from stomach pain for the whole day. Which material among the following could be used to relieve his pain?
A:dilute hydrochloric acid B: cucumber C:lemon
D:tamarind E:blueberries
- Electronegativity increases from left to right across the period in the periodic table. In which group and period does the most electronegative element belong?
A:group I period 3B:group VII period 2 C:group I period 7
D: group VII period 1E:group VII period 3
- During sunny days, the water in ponds dry completely leaves ponds barely. Which process takes place in that season?
A:condensationB:meltingC:evaporation
D:sublimationE:deposition
- The apparatus used to heat small amounts of solid substances within a gas jar is:
A:evaporating dishB:test tube holderC:deflagrating spoon
D:gas jarE:desiccator
- One of the following is a common component that causes reddish brown colour on some materials:
A:sodium metalB:alloyC:water vapour
D:oilE:grease
- Kileo visited the forest at their village, and fortunately, he found some water in the pond mixed with some dust particles. Which simple method among the following he used to get pure drinking water?
A:fractional distillationB:filtrationC:condensation
D:crystallizationE:simple distillation
- Ammonium ion reacts with sulphate ion to form a compound. The oxidation state of ammonium ion in that compound is:
A:+1B:4C:-1D:+4E:-2
- Asha boils the water from the well using electric kettle;but the heating process takes long time. The substance that causes this problem is:
A:aluminiumB:calciumC:sodium
D:potassiumE:both A and C
- Match the items from list A with the correct responses in list B by writing the letter of the correct response in your answer sheets.
| List A | List B |
| A:Electrovalent compound B:Potassium C:Bromine D:Covalent compound E:Phosphate F:Noble gases G:Metalloids |
SECTION B (70 Marks)
Answerall questions in this section
- Element X found in group II period 4 chemically interacted with element Y found in group VII period 3 forming compound Z.
(a)(i)Write the actual names of element X, Y and compound Z.
(ii)What is the chemical combination involved in this interaction?
(b)(i)Draw the structure of the compound Z
(ii)Give two properties of compound Z.
- (a) Air is a homogeneous mixture of different gases in the atmosphere. Give
three reasons to support this statement.
(b)With the help of balanced chemical equation, explain what will happen to:
(i)A piece of iron bar left to the exposure.
(ii)Anhydrous copper (II) sulphate when put into the watch glass and placed
on the laboratory bench.
- (a) (i) Why chemical symbols are very important to chemist? Give three reasons
(ii)Write the symbols of phosphorous, fluorine, manganese and copper.
(b)Why some elements are assigned symbols with only one letter while
Others bear with two letters?
- A certain compound was found to have the following composition by mass: 24.24% carbon, 4.04% hydrogen and 71.72% chlorine.
- What is the simplest formula of the compound formed?
- Calculate the percentage composition by mass of water in magnesium
Chloride hexahydrate.
- (a) (i) Give two reasons why laboratory exists are advised to open outward?
(ii)Why laboratory safety precaution is very important?
(b)Categorize the following laboratory compounds into corrosive and flammable:
Sodium hydroxide, spirit, sulphuric acid, oil, aro and benzene
- (a) Differentiate molar mass of a substance from molar volume of gases.
- The Golden Boy conducted an experiment for the production of oxygen gas
bythermal decomposition of potassium chlorate. If he used 20g of potassium
chlorate, what volume of oxygen would be produced at s.t.p?
- (a) Briefly state two importance of balancing chemical equations.
- Silver nitrate was introduced into dilute hydrochloric acid to form
products.
- Which type of chemical reaction took place?
- Write the net ionic equation for that reaction.
- (a) Give the names of the processes of making coke from coal and charcoal from
wood.
(b)(i)“Liquid fuel is more advantageous than solid fuel”. Give three points to
support this statement.
(ii)Write down the composition of water gas and producer gas.
- (a) A solution of sodium hydroxide was electrolysed using platinum electrodes.
Write the reactions which take place at the electrodes and give reason why
the solution becomes alkaline.
- What mass of zinc will be formed in electrolysis using a 15 amperes of
electricity for one and a half hours?
- Form three students at Tusomeni Secondary School performed an experiment for the neutralization of sodium hydroxide solution and hydrochloric acid. 25
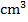 of sodium hydroxide were exactly neutralized by 25
of sodium hydroxide were exactly neutralized by 25 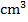 of 0.10M HCl.
of 0.10M HCl.
- Calculate the concentration of sodium hydroxide in:
- mol/
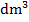 (ii) g/
(ii) g/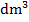
- What is the suitable indicator for:
- The above titration
- The titration of strong acid against strong base
SECTION C (15 Marks)
Answerone (1) question in this section.
- Cutting down trees for firewood and charcoal causes major catastrophic effects to the environment. Using four points analyse these effects and suggest two alternative ways that can be used to minimize the energy loss encountered.
- Water has a property of dissolving some minerals that affect it permanently; as a result, it becomes very disadvantageous to many rural people. As a young chemist and one among these people, explain the four effects of using this water and suggest two ways of removing the effect.
Page1 of5
FORM THREE CHEMISTRY EXAM SERIES 96
FORM THREE CHEMISTRY EXAM SERIES 96
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCE BASED SECONDARY EXAMINATION SERIES
CHEMISTRY 1MID TERMEXAMINATION
FORM THREE-AUGUST/SEPT-2021
Time: 3Hours
Instructions.
- This paper consists of section A, B and C with a total of 14 questions
- Answer all questions in section A and B and ONE (1) question from section C.
- Section A and C carries 15 marks, while section B 70 marks
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Non programmable calculators may be used.
- Write your number on every page of your answer booklet.
- Where necessary the following constants may be used;
Atomic masses; H=1, C=12, N=14,O=16, Na=23, S,=32, Ca =40, Cl =35.5, Cu=64, Zn=65.
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4dm3
1 faraday = 96,500 coulombs.
Standard temperature = 273K
Standard pressure = 760mmHg.
1 Litre = 1 dm3 = 1000cm3
SECTION A (15 Marks)
Answer All questions in this section.
1. For each of the items (i)-(xv), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i)Why oxygen differs from other gases?
- It neither burns nor support combustion.
- It supports combustion but does not burn.
- It burns but does not support combustion.
- It burns and supports combustion.
- It explodes and support combustion.
(ii) Why is hydrogen gas collected over water and by upward delivery method?
- It is insoluble in water and less denser than air.
- It is soluble in water and denser than air.
- It is insoluble in water and denser than air.
- It is soluble in water and less denser than air.
- It is soluble in both water and air.
(iii) The following are the uses of chromatography except:
- to analyse blood in crime scenes.
- to detect different fibres.
- to detect water pollution.
- to bleach dye/colour.
- to test purity of organic substances.
(iv) Which statement is the most correct about chemistry laboratory?
- Is a special room designed for conducting chemical tests.
- Is a special room designed for science practicals.
- Is a special room designed for keeping apparatuses.
- Is a special room where data analysis is carried out.
- Is a special room where students learn chemistry.
(v) Which carbonate is the most stable to heat?
- Calcium carbonate
- Copper (II) carbonate
- Lead (II) carbonate
- Zinc carbonate
- .Iron (II) carbonate.
(vi) In the following equilibrium equation, 2S02(g) +O2(g) ![]() 2S03 The forward reaction is exothermic. Which change would increase the production of sulphur trioxide at equilibrium?
2S03 The forward reaction is exothermic. Which change would increase the production of sulphur trioxide at equilibrium?
- Increasing temperature
- Decreasing temperature
- Decreasing sulphur trioxide concentration
- Decreasing pressure
- Adding a catalyst.
(vii) Which of these can be reduced when heated with carbon?
- Aluminium
- Calcium carbonate
- Iron (III) oxide
- Magnesium oxide
- Sodium oxide.
(viii) Which of the following is the electronic configuration of an element Y found in period 3 and group II of the periodic table?
- 2:8
- 2:8:2
- 2:6
- 2:8:8:2
- 2:8:4
(ix) Which of the following is NOT among the composition of air?
- Noble gases
- Carbon dioxide
- Nitrogen
- Hydrogen
- Water vapour.
(x) If a stead current of 2 amperes was passed through an aqueous solution of iron (II) sulphate for 15 minutes, then the mass of iron deposited at the cathode will be:
- 54 g.
- 56 g.
- 0.54 g.
- 28 g.
- 0.52 g.
2. Match the items in List A which the responses in List B by writing the letter of the correct response beside the item number in the answer booklet provided.
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. An atom of element X having atomic number 11 combines with an atom of element Y haying atomic number 9 to form a compound.
(a) Write the formula of the compound and state the type of bond formed in the compound.
(b) Give four properties of the compound formed in 7(a). (7 marks)
(b)
- Soluble in water
- High melting and boiling point
- Contact electricity in molten state
- Consists of ionic structure.
4. Explain how to handle chemicals having the warning signs of flammable, corrosive, harmful, explosive and toxic in the laboratory.
5. (a) Copper obtained from copper pyrites (CuFeS2) is impure for electrical wiring and has to be purified by electrolysis.
(i) Name the electrolyte and the electrodes used during electrolysis.
(ii) Write the observations that can be made during the electrolysis.
(b) The following flow diagram shows the stages in the contact process 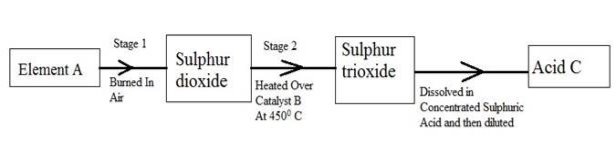
(i) Give the names of element A, catalyst B and an acid C.
(ii) Write a balanced chemical equation for the formation of sulphur trioxide in stage 2
6. (a) Copper can be obtained from the ore, copper pyrites (CuFeS2). The ore is heated in a limited amount of air giving the following reaction:
4CuFeS2 + 11O 2 ? 4Cu + 2Fe 2 O 3 + 8SO2 .
(i) Calculate the maximum mass of copper that can be obtained from 367 kg of copper pyrites.
(ii) State why the gaseous product from this reaction must not be allowed to escape into the atmosphere.
(b) State three industrial application of electrolysis.
7. A student attempted to prepare hydrogen gas by reacting zinc metal with dilute sulphuric acid. In this experiment zinc metal granules of about 0.5 cm diameter and 0.20 moles of acid were used.
The rate of formation of hydrogen gas was found to be slow.
(a)Explain three ways in which the rate of formation of hydrogen gas could be increased.
(b)If the student wanted 36 cm3 of hydrogen gas at s.t.p, what amount of the acid would be required.
8. (a) 20 cm3 of a solution containing 7 g dm-3 of sodium hydroxide were exactly neutralized by 25 cm3 of 0.10 M hydrochloric acid. Calculate the concentration of sodium hydroxide in moles per dm3.
(b) Give two examples in each of the following solution.
(i) Gaseous solution.
(ii) Solid solution.
9. The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
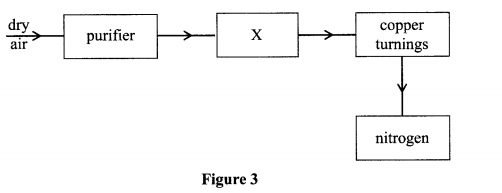
(a) Identify X (I mark)
(b) Write an equation for the reaction with heated copper turnings. (1 mark)
(c) Name an impurity in the sample of nitrogen gas. ( I mark)
10. (a) Name two ores in which sodium occurs.
(b) During extraction of sodium using the down's process, calcium chloride is added to the ore. Give a reason for the addition of calcium chloride. (1 mark)
(c) State two uses of sodium. ( I mark)
11. Figure 3 shows the apparatus used to burn hydrogen in air. Use it to answer the questions that follow.
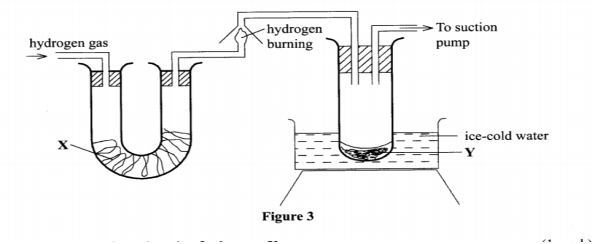
State the role of substance X.
(ii) Give the name of the substance that could be used as X. (1 mark)
(iii) State the role of the suction pump. (1 mark)
(iv) Name the product Y formed. (1 mark)
(v) Give a simple physical test to prove the identity of Y. (1 mark)
(vi) State the difference between 'dry' and 'anhydrous'. (2 marks)
12. (a) Consider elements with atomic number 1, 11, 12 and 17.
(i) What are the types of oxides formed by elements with atomic number 11 and 12?
(ii) Write an equation which represents a reaction between the element with atomic number 1 and 17.
(iii) Write a balanced chemical equation between the oxide of the element with atomic number 11 and aqueous solution of the compound formed in 4 (a) (ii).
(b) Suggest one method for the separation of each of the following:
(i) Iodine and sand.
(ii) Green solution from leaves.
(iii) Alcohol and water.
(iv) Iron fillings and powdered calcium carbonate.
SECTION C (15 Marks)
Answer one (1) question in this section.
13. 25 cm3 of 0.1 M HCl were neutralized by 23 cm3 of sodium hydroxide solution. Calculate the concentration of the alkali in grams per litre.
14. Describe four common stages for the extraction of metals. Does the extraction of gold follow all four stages? Give reasons.
FORM THREE CHEMISTRY EXAM SERIES 58
FORM THREE CHEMISTRY EXAM SERIES 58
THE PRESIDENT'S OFFICE
MINISTRY OF REGIONAL GOVERNMENT AND LOCAL GOVERNMENT
AUGUST-SEPTEMBER EXAMINATION SERIES
CHEMISTRY FORM-3
2020
TIME: 2:30 HRS
Instructions
- This paper consists of sections A, B and C with a total of fourteen (14) questions.
- Answer all questions in sections A and B and one (1) question from section C.
- Cellular phones and any unauthorised materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet(s).
- The following constants may be used.
Atomic masses: H 1, O- 16, N- 14, S = 32, Zn - 65, Cl -35.5, cu - 64.
Avogadros number= 6.02 x 1023 ![]()
GMV at s.t.p =22.4 dm3 .
1 Faraday= 96,500 coulombs.
Standard pressure = 760 mm Hg. Standard temperature 273 K.
1 litre =1 dm3 =1000 cm 3.
SECTION A (15 Marks)
Answer all questions in this section.
1. For each of the items (i) — (x), choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) "Water is referred to as the universal solvent". What does this mean?
- Water is neither acidic nor basic as compared to other liquids.
- Water exists in three states of matter than any other liquids.
- Water dissolves both organic and inorganic solutes.
- Water is used more domestically than any other liquids.
- Water dissolves more substances than any other known liquids.
(ii) What is the proper set of apparatus would you use to grind granules of a solid substance into fine powder in the laboratory?
- Pestle and filter funnel
- Separating funnel and mortar
- Pestle and filter paper
- Pestle and mortar
- Thistle funnel and mortar
(iii) Which of the following sets of processes uses a gas that ignites with a "pop" sound when a lighted splint is passed through it?
- Balloon filling, welding and diving
- Hardening oil, balloon filling and welding
- Hardening oil, balloon filling and diving
- Fueling rocket, diving and welding
- Balloon filling, fueling rocket and diving
(iv) A current of 0.2 A was passed through an electrolyte for 16 minutes and 40 seconds. What is the quantity of electricity produced in coulombs?
- 2000 C
- 1000 C
- 200 C
- 0.20 C
- 7686 C.
(v) Aluminium does not react with water and does not corrode much in air because
- it is below hydrogen in the reactivity series
- it forms a stable carbonate which prevents reactions
- the metal is covered with a protective coating of an oxide
- aluminium ions have positive charges
- it is very stable.
(vi) When a burning fuel produces blue color it means there is
- adequate supply of oxygen with production of soot.
- inadequate supply of oxygen without production of soot.
- inadequate supply of oxygen with production of soot.
- adequate supply of oxygen with production of less heat.
- adequate supply of oxygen with production of more heat.
(vii) Which of these can be reduced when heated with carbon?
- Aluminium
- Calcium carbonate
- Iron (III) oxide
- Magnesium oxide
- Sodium oxide.
(viii) Which of the following is NOT among the composition of air?
- Noble gases
- Carbon dioxide
- Nitrogen
- Hydrogen
- Water vapour.
(ix) If a steady current of 2 amperes was passed through an aqueous solution of iron (II) sulphate for 15 minutes, the mass of iron deposited at the cathode will be.
- 30g.
- 56g.
- 0.54g.
- 28g.
- 0.52g.
(x) Two substances are allotropes of carbon if
- Both reduce heated iron (II) oxide to iron
- Have different crystalline structure
- Have equal masses
- Have equal shape
- Have the same arrangement of atoms
2. Match the descriptions in List A with the corresponding scientific procedures in List B by writing the letter of the correct response besides the item number in the answer booklet provided.
| LIST 1 | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. (a) How many chlorine molecules are in 20 cm of chlorine gas at s.t.p?
(b)Calculate the number of ions present in 5 g of copper II nitrate.
4. (a) Distinguish temporary hardness from permanent hardness of water.
(b) With the help of chemical equations, explain how you can remove each type of water hardness in 5(a).
5. (a) Copper obtained from copper pyrites (CuFeS2) is impure for electrical wiring and has to be purified by electrolysis.
(i) Name the electrolyte and the electrodes used during electrolysis.
(ii) Write the observations that can be made during the electrolysis.
(b) The following flow diagram shows the stages in the contact process 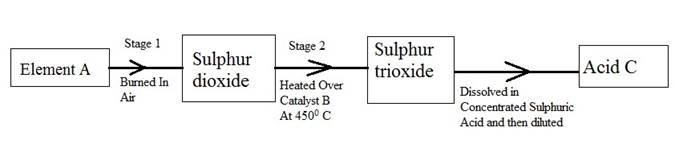
(i) Give the names of element A, catalyst B and an acid C.
(ii) Write a balanced chemical equation for the formation of sulphur trioxide in stage 2
6. (a) Give one example in each of the following:
(i) Alkali earth metals.
(ii) Noblegases .
(iii) Transition elements.
(b) Write the names of the following processes of changing matter from one state to another.
(i) Gas to liquid.
(ii) Ga s to solid.
(iii) Solid t o gas .
7. (a) State four steps employed in the extraction of moderate reactive metals.
(b) Write balanced chemical equations to show how chlorine reacts with the following:
- water.
- aqueous iron (II) chloride solution.
- hydrogen sulphide.
8. (a) State three main physical properties of water and show the usefulness of each property.
(b) State three industrial application of electrolysis.
9. (a)An atom M has an atomic number 14 and mass number 28.
(i)What is the number of protons and neutrons?
(ii) Write the electronic configuration of atom M.
(b) Calculate the volume of water which was produced when 1,120 cm3 of oxygen at s.t.p. was liberated during the decomposition of hydrogen peroxide. The density of water = 1.0 g/cm3
10. (a) Determine the empirical formula of a substance that has the following composition by mass; 49.5% oxygen.
(b) Give one reason why Alluminium is chosen to make each of the following items:
- Cooking foil
- Overhead electric cables
- Window frames
11. (a) Identify and state the environmental problem caused by the gas which is released from the blast furnace in the extraction of iron from its oxide.
(b) (i) Draw a labeled diagram of a simple electrolytic cell which show how copper is purified.
(ii) Write balanced ionic equations to show the electrode reactions which occur when copper is purified.
12. (a) (i) Why chemistry laboratory exits open outward?
(ii) State the uses of any four items found in a First Aid Kit.
(b) (i) Arrange the following metals in order of increasing reactivity; zinc, magnesium, calcium, copper and mercury.
(ii) Which one of the metals in (b) (i) above reacts with steam to form an oxide which is white when cold and yellow when hot?
SECTION C (15 Marks)
Answer one (1) question from this section.
13. In Tanzania, soil conservation is very important for Industrial Materials production. Explain six methods that are used to manage loss of plant nutrients from the soil.
14. 0.48g of a metal, M was placed in a test tube and hot copper (II) sulphate solution was added to it and stirred until the reaction stopped. The metal (M) displaced copper from copper (II) sulphate solution. Copper was filtered, washed with water, dried at 1000 C and the mass found to be 1.27g. Given that, the balanced chemical reaction that occurred is M (s) + CuSO 4(aq)  MSO 4(aq) + Cu (s)
MSO 4(aq) + Cu (s)
(a) Calculate;
- The number of moles of copper that were formed and the number of moles of M that were used in the reaction.
- The relative atomic mass of M and hence identify metal M.
(b) State the appearance of the metal formed (Cu).
(c) With ionic equations, explain why the reaction can be considered to involve both oxidation and reduction.
FORM THREE CHEMISTRY EXAM SERIES 24
FORM THREE CHEMISTRY EXAM SERIES 24
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256