THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
CHEMISTRY FORM FOUR
TIME: 2 HOURS NOVEMBER 2022
INSTRUCTIONS:
1. This paper consists of sections A, B and C
2. Answer all questions in section A and B and only one (01) question from section C
3. Cellular phones and all programmable calculators are not allowed in the examination room
4. Write your examination Number on every page of your answer booklet(s)
5. The following constants may be used
1 Faraday = 96500 coulombs
G.M.V at stp = 22.4dm 3
Avogadro's number = 6.02 x 10 23 particles per mole Atomic masses
H = 1, C = 12, O = 16, Na = 23, S = 32, cU = 64, Ag = 108, Zn = 65
SECTION A: (15 MARKS)
Answer all questions in this section
1. For each of the items (i) — (x), choose the most correct answer from among the given alternatives and write its letter in the space provided
(i) The lead in a pencil is made of a mixture of graphite and clay. When the percentage of graphite is increased, the pencil moves across the paper move easily. Which statement explain this observation?
A. Graphite has high melting point
B. Graphite is a lubricant
C. Graphite is the form of carbon
D. Graphite is a non — metal
E. Graphite is a good conductor of electricity
(ii) Which process is used to convert calcium carbonate into calcium oxide
A. Electrolysis
B. Fractional distillation ![]()
C. Cracking
D. Thermal decomposition
E. Evaporation
(iii) One of the following substance is commonly used to determine the arrangement of metals in order or reactivity
A. Water
B. Salt
C. Acid
D. Base
E. Non -metal
(iv)The hottest part of a non - luminous flame is the region of
A. Green or blue zone
B. Purple or blue zone
C. Colourless inner zone
D. Thin outer zone
E. Blue zone
(v) The following are the properties of a good fuel except
A. Average value of velocity or combustion
B. High ignition point
C. Highest energy value
D. Environmental friends
E. Less or no waste products
(vi) A sodium atom (Na) and a sodium iron (Na+) have the same
A. Atomic site
B. Electronic configuration
C. Physical properties
D. Physical properties
E. Number of protons
(vii)Factors in an experiment that remain unchanged throughout the experiment is known as
A. Controlled variable
B. Dependent variable
C. Independent variable
D. Uncontrolled variable
E. Fixed factor
(viii) Coal, methane and hydrogen are burned as fuel, which description of this process is correct?
| What happens to the fuel | Type of reaction |
| Endothermic Exothermic Endothermic Exothermic Displacement |
(ix)Petroleum is a mixture of different hydrocarbons, which process is used to separate petroleum into groups of similar hydrocarbons?
A. Combustion
B. Cracking
C. Fractional distillation
D. Reduction
E. Isomerism
(x) A car releases 5g of carbondioxide gas into air for each mile driven. The number of carbondioxide molecules released are
A. 1.0 x 1023
B. 6.8 x 1023
C. 6.3 x 1023
D. 5.8 x 1022
E. 6.8 x 1022
2. Match the item in LIST A with a correct response in LIST B by writing the letter of tie correct response below the corresponding item in the table G![]() iven
iven
| LIST A | LIST B |
|
|
SECTION B: (70 MARKS)
Answer all questions in this section
3. (a) Element Q has 17 electron and 18 neutrons (i) What is the atomic number of element Q
(ii) What is the mass number of element Q
(iii) Write down the electronic configuration of element Q
(iv) In which group and period in the periodic table does element Q occupy?
(b)Given the following 204J 208 K 207L and AM are isotopes of element H, whose abundances are 2%, 24%, 22% and X% respectively. Calculate the abundance X% and the mass number A of isotope M, given that the atomic mass of element H is 207.
4. (a) State any three main physical properties of water and show the usefulness of each property.
(b)The following methods were used to soften 25cm 3 of the original sample of water. At the end of each experiment, the resultant product was treated with soap solution until lather was obtained
| Method used | Volume required for 25cm3 0f product |
| Filtering | 20 |
| Distilling. | 1 |
| Adding Na2CO3 washing soda | 1 |
| Boiling | 20 |
| Adding zeolite | 1 |
(i) Which methods were most suitable for softening hard water?
(ii) Which type of hardness was present in the sample provided? Explain
(iii) Which substances caused the hardness in the sample water?
(iv) Why did distillation and filtration produce such different results?
5. (a) Outline three reasons to explain why carbondioxide is used to extinguish fire
(b)A sample of mass 28.6g of hydrated sodium carbonate (Na2 C03. 101420) was heated such that water was entirely absorbed by 32g of anhydrous copper (Il) sulphate to form a blue compound of hydrated copper (Il) sulphate (CUS04 . XH20). Find the value of x in the copper (Il) sulphate
6. (a) Complete and balance the following chemical equation.
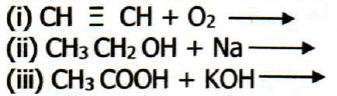
(b) Write down the condensed structural isomers of alcohol of molecular formula C4H100
7. (a) By using two examples in each case, categorize fuel according to their physical state
(b) What is the simplest chemical formula of a compound formed when 36g of magnesium combine with 14g of nitrogen?
8. (a) Complete the following table
| Element | When burned forms | Kind of oxide | Action of oxidation water | Action on litmus |
| Carbon | Co2 |
|
|
|
| Sodium |
|
| NaoH |
|
| Sulphur |
| Acidic |
| Blue to red |
(b) Using calcium oxide and carbon dioxide explain the meaning of
(i) Acidic oxide (ii) Basic oxide
9. A piece of marble chips (calcium carbonate) was placed in a beaker containing an excess of hydrochloric acid standing on a reading balance. The mass of the beaker and its contents were recorded after eve 2 minutes as shown in the diagram below
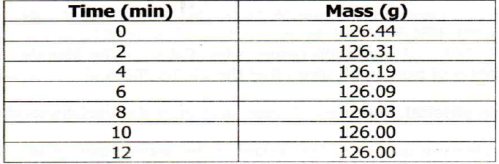
(a) Why was there a loss of mass?
(b) Write the equation for the reaction above
(c) State three (3) different ways in which reaction could have been more rapid
![]() (d) Why did the mass remain constant after 10 minutes?
(d) Why did the mass remain constant after 10 minutes?
10. (a) Nowadays the use of spatula which are made of iron is discouraged in the laboratory. Explain one reason for the scenario.
(b) Write a chemical composition for the following fire extinguisher
(i) Dry powder (ii) Wet chemical
(c) How can you prevent the following iron materials from rusting?
(i) Gates
(ii) Bicycle chain
(iii) Iron sheets
11. (a) How can a mixture of two or more miscible liquids be separated from one another?
(b) Make a flow chart to show the separation of a mixture of common salt and sand
12. Four experiment were conducted using chemicals P, Q, L and M on different materials.
The results were given as indicated below. You are required to write the correct terminology which suits each of the results below and give precautions of handling the warning signs which goes with it.![]()
(i) When chemical P was poured on the wood it completely damage the surface of the wood
(ii) When chemical Q was poured on the small fire, it found burst and turning a bigger fire
(iii)When chemical L was swallowed by a rat, the rat died immediately
(iv)When chemical M was exposed on air sudden blast accompanied with light occurred (b) Give one main importance of chemical warning signs
SECTION C: (15 MARKS)
13. (a) State Faraday's first law of electrolysis
(b)The apparatus shown below was used in the experiment of electroplating a key with silver. The electrolyte used is silver nitrate and an electric current of 15A was passed in the electrolyte for two and half hours. Study it carefully and answer the questions that follows;
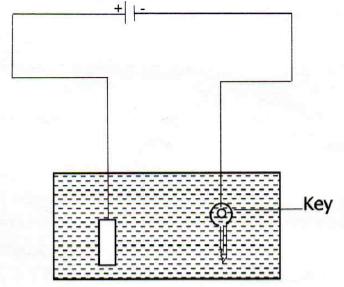
(i) Name the process taking place on key as either oxidation or reduction
(ii) With the current of 15A, what mass of silver was deposited on the key?
(iii) Name the gas formed at the anode![]()
(iv) Name the acid formed as a result of electrolyzing silver nitrate
(v) What volume of the named gas in (iii) above was collected in this experiment?
14. (a) Eutrophication is excessive growth of aquatic plant and algae in water bodies (i) Give two causes of eutrophication
(ii) Explain the effect of eutrophication. (Give two points)
(b)Explain how industrial workers can be protected against harmful effect of the chemicals. (Give three points)
FORM FOUR CHEMISTRY EXAM SERIES 129
FORM FOUR CHEMISTRY EXAM SERIES 129
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256







