PRESIDENT OFFICE REGIONAL ADMNISTRATIONAND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
COMPETENCE BASED ASSEMENT
032/1CHEMISTRY FORM THREE
ANNUAL EXAMINATIONS – 2023
Time: 3 Hours
Instructions
- This paper consists of section A, B and C with a total of thirteen (13) questions.
- Answer all questions in this paper
- Calculators, cellular phones and any unauthorized materials are not allowed in the examination room
- Write your Examination Numberon every page of your answer booklet(s)
- The following constants may be used
Atomic masses:
H=1,C=12,O=16,N=14,Ag=108
Avogadro’s number = 6.02 x 1023
GMV at s.t.p = 22.4 dm3
1 Faraday = 96,500 coulombs
Standard pressure = 760 mm Hg
Standard temperature = 273 K
1 litre = 1dm3= 1000cm3
SECTION A. (16 MARKS)
- For each of the items (i) – (x), Choose the correct the answer from among alternatives given and write its letter in the answer sheet provided
- Loose or floppy clothing is not allowed in the laboratory because;
- Movement has to be fast
- It will get wet when water splashes
- It may catch fire or cause one to fall
- It cause poor ventilation in the body
- It prevents experiment from being conducted well
- What mass of Suphuric acid (H2SO4) is found in 400cm3 of its 0.1M?
- 2.67g
- 9.8g
- 4.89g
- 3.92g
- 8.69g
- One of the Isotopes of an element X has atomic number Z and a mass number A. What is the number of Neutron contained in Nucleus of the element?
- Z – A
- A
- A + Z
- Z
- A – Z
- A solution of PH 5 is said to be
- Strong base
- Neutral
- A weak acid
- A strong acid
- A weak base
- In an experiment, 1930 coulombs Liberated 0.64g of copper when the same quantity of electricity. Was passed through a solution of silver Nitrate. What amount of silver was deposited?
- 32g
- 2.16g
- 10.8g
- 108g
- 21.6g
- In blast furnace carbon monoxide in prepared by passing carbon dioxide over a red hot coke. What is the chemical role of carbon dioxide
- An accelerator
- An oxidizing agent
- A catalyst
- A reducing agent
- Flammable
- The following shows four uses of iron, in which of these uses are the iron most likely to rust?
- Iron bucket electroplated with zinc
- Iron wired Alluminium electric cable
- Iron hinges on gate
- Alloyed piston
- Panted iron gate
- Alluminium does not react with water and does not corrode much in air. Why?
- It is below hydrogen in reactivity series
- It forms a stable carbonate which prevent reactions
- The metal in coated with a protective coating of Oxide
- It is very unstable
- Does not react with water
- When bumming fuel produce blue colour, it means there is
- Adequate supply of Oxygen without production of soot
- Inadequate supply of Oxygen without production of soot
- Inadequate supply of Oxygen with production of soot
- Adequate supply of Oxygen with production of more heat
- Inadequate supply of Oxygen with production of more heat
- The reason why white anhydrous copper II sulphate tums blue when exposed in atmosphere is that it.
- Absorb moisture
- React with Oxygen
- React with carbon dioxide
- Becomes dry
- Release water to atmosphere.
- Match items in LIST A with correct response in LIST B by writing the letter of correct response beside the item number in answer sheet provided.
| LIST A | LIST B |
|
|
SECTION B. (54 Marks)
Attempt all questions in this section
- (a)Explain how the following differ from one another
- A base and Alkali
- An atom and isotope
(b) An organic compound P consists of 52.2% carbon 13% hydrogen and 34.8% Oxygen. The vapour density of P is 23. Calculate molecular formula of the compound.
(c) Calculate Oxidation number of Nitrogen in potassium nitrate.
- (a)Give three applications of separation of mixture in our daily life.
(b) 20.0cm3of sodium hydroxide containing 8.0gdm3was required for complete neutralization of 0.18g of dibasic acid. Calculate the relative molecular mass of the acid
- (a)State faraday’s Laws of electrolysis
(b) Dilute Silver Nitrate solution was decomposed by passage of electric current through it. What mass of Silver and what volume of Oxygen (measured at (STP) would be liberated in electrolysis by 9650C of electricity?
- 5.3g of X2CO3 was dissolved in water to make 0.5 litre of a solution 25cm3 of this solution required 50cm3 of 0.1M HCl for complete reaction.
- Write a balanced chemical reaction for complete neutralization
- Calculate concentration of X2CO3 in moles dm-3
- What is the relative molecular mass of X2CO3
- Give the name of element X
- (a)What do you understand by the following terms
- Mole concept
- Molar volume of gas
(b)Consider the equation below for dissociation of Suphuric acid
H2SO4(aq)→ 2H(aq)++ SO42-(aq)
From equation, how many ions are there in 9.8g of sulphuric acid?
- (a)Consider the following elements
16O8,19F9,20Ne1023Na1124Mg12. Atoms and ions of these elements can be 150-electronic (have same number of electrons)
- Write down their symbols when they are ISO-electrode
- Write down their common electronic arrangement in their ions and atoms
(b)Although Sulphur dioxide is an Oxide, It can be further Oxidized
- Write an equation and Condition for further Oxidation of sulphur dioxide
- Write down an equation showing how the product can be used industrially to obtain desired Materials to be produced.
- Give the name for this industrial process
- Write two uses of product formed by industrial process named in (iii) above
SECTION C (30 Marks)
Answerany twoquestions from this section
- (a)Describe the extraction of sodium from its ore and Write all the reaction equation
(b) State four uses of sodium metal
- (a) Preventing rusting, you should prevent contamination of water and air with iron and steel, also to avoid using material made from iron or steel. State the method that can be used to prevent rusting on each of the following
- Iron sheet
- Bridge and pipes
- Ship
- Machine parts
(b) Fire extinguisher is used to stop fire. List five types of fire extinguishers
- (a)Briefly explain the following observation about a sample of hard water
- When boils it forms white precipitate
- After boiling, water forms a scum
- Sodium carbonate makes the water completely soft
(b) With the acid of chemical equation, briefly describe the following processes
- The removal of temporary hardness of water by boiling
- The removal of permanent hardness of water by chemical means
FORM THREE CHEMISTRY EXAM SERIES 158
FORM THREE CHEMISTRY EXAM SERIES 158
THE PRESIDENT’S OFFICE MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
COMPETENCY BASED SECONDARY EXAMINATION SERIES
032/1 CHEMISTRY FORM THREE
TIME: 2 HOURS NOVEMBER 2022
INSTRUCTIONS
- This Paper Consists of Sections A,B nd C
- Answer all questions in section A and B and only one(1) question from section C
- Cellular phone and any unauthorized material are not allowed in examination room
- Write your Examination Number on every page of your answer sheet(s).
- The following constant may be useful
- Atomic masses H=1, C=12, N=14,O=16,Na=23,Mg=24,S=32,Cu=64.
- Avogadro’s number = 6.02 6.02 x 10 23
- G.M.V at S.T.P = 22.4dm 3
- 1Faraday = 96500coulombs
- 1litre = 1dm3 = 1000cm 3
SECTION A. (20 MARKS)
1. For each items (i)-(x) choose the correct answer among the given alternative and write the letter beside the item number in the booklet provided
(i) Which of the following is the agricultural product made by the application of chemistry?
- Yeast D. Pesticide
- Drug E. Cement
- Clothes
(ii) When methane undergo substitution reaction with excess chlorine .what is the end product
- Chloromethane D. Monochloromethane
- Dichloromethane E. Tetra chloromethane
- Dichloromethane
(iii) An element X is found in period 4; group II of the periodic table .if the element X Undergo the reaction X→X 2+ +2e - ; the electronic configuration of X ion formed will be A. 2: 8:6 B. 2:6 C. 2:8:4 D. 2:8:8:2 E. 2:8:8
(iv) A current of 0.2A was passed through an electrolyte for 16 minutes and 40 seconds .what is the quantity of electricity produced in coulombs.
A. 2000C B. 1000C C. 200C D. 0.2C E. 7686C
(v) Which carbonate is the most stable to heat?
- Calcium carbonate D. Zinc Carbonate C. Lead II carbonate E. Iron carbonate
- Copper II carbonate
(vi) Skin injury that cause a change in the colour of skin
- Bruises B. Bum C. Scalds D. Shock E. Suffocation
(vii) A good fuel is the one which has.
- High speed of continuous energy supply D. High carbon dioxide production
- High energy value supplied E. High content of non-combustible materials
- Low carbon dioxide production
(viii) The region of the atmosphere which contains the ozone layer is called
- Mesosphere D. Troposphere
- Stratosphere E. Metamosphere
- Atmosphere
(ix) Aluminum is said to be amphoteric oxide because
- It acts as an acid and also It acts as a base
- It acts as a neutral compound
- It acts as strong reducing agent
- It acts as a basic compound
(x) The main impurities in Nitrogen gas prepared in the laboratory are:-
- Water vopour D. Dust particles
- Oxygen E. Noble gases
- Carbondioxide
2. Match the item in LIST A with a correct response in LIST B by writing the letter of the response below the corresponding item in the table given
| LIST A | LIST B |
| (i) A gas that forms explosive mixture with air and water (ii) A gas that forms a reddish brown fumes when comes in contact with air (iii) A gas that turns lime water milky (iv) A colorless gas that bleaches moist coloured flower (v) Only basic gas |
|
SECTION B. (70MARK)
Answer All questions in this section
3. (a) A stone is said to be a good example of matter. Give two reasons to support this fact.
- Outline two significance of chemical symbols of an element.
- Explain by giving reasons why:
- Laboratory door open outwards
- Laboratory floor is rough and never polished
- Fume chamber is important in chemistry laboratory
4. (a) Identify the substances by using the following information.
- A solid is yellow when hot and white when cold
- A colorless gas turns a yellow acidified potassium dichromate paper to green
- When water is added to a white powder, the white powder changes to blue crystals
- With the aid of an equation in each case, explain what will be observed when
- Chlorine gas is bubbled through a solution of iron II chloride
- Hydrogen Chloride gas is passed through a jar containing ammonia gas.
- A piece of sodium metal is dipped into a beaker of water containing some red litmus paper
- Carbondioxide is blown into a test tube filled with lime water.
5. (a) outline three reasons to explain why Carbondioxide is used to extinguish fir
(b) A sample of mass 28.6g of hydrated sodium carbonate ( Na 2 CO 3 .10H 2 O) was heated such that; its water was entily absorbed by 32g of anhydrous copper II sulphate to form a blue compound of hydrated( CuSO 4 .XH 2 O) .find the value of X in the copper sulphate.
6.(a)A student aimed to prepared a gas X by reacting a moderate reactive metal with a dilute acid .use this information to answer the following question
- What is the name of the gas X
- What is the test of the gas X
- With reasons; state the appropriate method of collecting gas X
- Write the balanced chemical equation for the reaction.
(b) State four properties that make Aluminium useful in overhead cables
7. (a) Calculate the morality of 5% by weight of a solution of sodium hydroxide
(b) What is the simplest chemical formula of a compound formed when 36g of Magnesium combine with 14g of Nitrogen?
8.. (a) During electrolysis of an aqueous solution of salt of metal M , a current of 2.0A was passed for 32minutes and 10 second .the mass of metal M deposited was 2.24g.
- On which electrode was the metal deposited
- Calculate the amount of charge needed to deposit 1mole of metal M
- Calculate the charge carried on the ion
- Write an ionic equation to show how the ion of metal M are discharged at the electrode (R.A.M of metal M is 112)
(b)During electrolysis of brine .Sodium is deposited at the cathode and chlorine gas is released at the anode .if 2.0g of sodium are collected at the cathode .find the volume of chlorine released at S.T.P.
9. (a) State the le-chatelier’s principle
- State what will happen in the process of equilibrium involving the equation
2SO 2 (g) + O 2 (g) ⥫⥬ 2SO 3 (g) ∆H = -94.4KJMol -1 If
- Temperature is lowered
- Pressure is increased
- The concentration of SO 3 is removed from the system.
(c)Briefly write one application of le- chatelier’s principle.
9. (a) States any three main physical properties of water and show the usefulness of each property
(b)During large – scale treatment of water, what two chemical are added at various stage?. Explain their use.
10. (a) i. Extraction of metal is said to be reduction process. Explain
ii. Why Sodium is collected by upwards in the down cell?
(b) Describe the use of each of the following during extraction of sodium
- Calcium chloride
- Steel gauze
- Name the ores commonly used in the extraction of iron metal
11. (a)State the modern periodic law
(b)Study the periodic table below then answer the questions that follow.
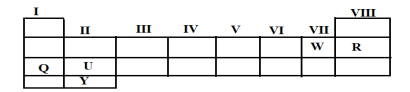
Write the formula of compounds formed when element
- U and W combine together
- Q and W combine together
Draw and write electronic configuration of Y.
12. (a)Catherine is planning to make fire for cooking ugali for her family. What are necessary conditions which must be present so that she can make fire successfully for cooking ugali for her family?
(b)Give a reason for each of the followings
- Water is universal solvent
- Some metal like zinc do not get rust.
- Chlorine gas is collected by downward delivery
- Carbon dioxide turns lime water into milky colour.
SECTION C (15 MARKS)
Answer only one (1) question.
13. (a)Give good reason (s) for the following. (Answer according to the question demand).
- Natural gas is so popular in heating and cooking in homes. (2 points)
- Nuclear energy is not a sustainable source of energy. (2 points)
- Coal and petroleum are non-renewable sources. (1 point)
(b) State the main raw materials and process involved in the manufacture of each of the following products.
- Wood charcoal
- Coke
- Lamp black
- Animal charcoal
14. (a) 25cm 3 of 0.1MHCl were neutralized by 23cm 3 of Na 2 CO 3 solution. Calculate the concentration of the alkali in grams per litre.
(b)Suggest a suitable indicator of each of the following titrations
- Hydrochloric acid against ammonia solution
- Sulphuric acid against sodium hydroxide solution
- Ethanedioic acid against potassium hydroxide solution
FORM THREE CHEMISTRY EXAM SERIES 104
FORM THREE CHEMISTRY EXAM SERIES 104
THE PRESIDENT’S OFFICE
MINISTRY OF EDUCATION, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
SECONDARY EXAMINATION SERIES
CHEMISTRY ANNUAL EXAMINATION
FORM THREE-NOVEMBER 2021
Instructions
- This paper consists of sections A, B and C with a total of fourteen (14) questions.
- Answer all questions in sections A and B and one (1) question from section C.
- Sections A and C carry fifteen (15) marks each and section B carries seventy (70) marks.
- Cellular phones and any unauthorised materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet(s).
- The following constants may be used.
H 1, C = 12, 0=16, N = 14, Cu = 64, Pb = 108.
A vogadros number = 6.02 x 1023.
GMV at s.t.p = 22.4 dm3.
1 Faraday = 96,500 coulombs.
Standard pressure = 760 mm Hg.
Standard temperature = 273 K.
1 litre = 1 dm3 = 1000 cm3.
SECTION A (20 Marks)
Answer all questions in this section.
1. For each of the items (i) (x), choose the correct answer from the given alternatives and write its letter beside the item number.
(i) Which of the following is an agricultural chemical products made by the application of chemistry?
- Drugs
- Pesticides
- Clothes
- Yeasts
- Cement.
(ii) A current of 0.2 A was passed through an electrolyte for 16 minutes and 40 seconds. What is the quantity of electricity produced in coulombs?
- 2000 C
- 1000 C
- 200 C
- 0.20 C
- 7686 C.
(iii) Substance X liberates chlorine gas from acidified potassium chloride. The behaviour of X is described as:
- an oxidising agent
- an oxidising and reducing agent
- catalyst
- a reducing agent
- bleaching agent.
(iv) Which carbonate is the most stable to heat?
- Calcium carbonate
- Copper (II) carbonate
- Lead (II) carbonate
- Zinc carbonate
- .Iron (II) carbonate.
(v) Aluminium does not react with water and does not corrode much in air because
- it is below hydrogen in the reactivity series
- it forms a stable carbonate which prevents reactions
- the metal is covered with a protective coating of an oxide
- aluminium ions have positive charges
- it is very stable.
(vi) Which of the following compounds does NOT belong to the alkenes homologous series?
- C2H4
- C3H6
- C4H 8
- C5H10
- C6H 14.
(vii) In the following equilibrium equation, 2S02(g) +O2(g) ![]() 2S03 The forward reaction is exothermic. Which change would increase the production of sulphur trioxide at equilibrium?
2S03 The forward reaction is exothermic. Which change would increase the production of sulphur trioxide at equilibrium?
- Increasing temperature
- Decreasing temperature
- Decreasing sulphur trioxide concentration
- Decreasing pressure
- Adding a catalyst.
(viii) When a burning fuel produces blue color it means there is
- adequate supply of oxygen with production of soot.
- inadequate supply of oxygen without production of soot.
- inadequate supply of oxygen with production of soot.
- adequate supply of oxygen with production of less heat.
- adequate supply of oxygen with production of more heat.
(ix) Which of the following equations represents the combustion of methane with the products collected at 120oC?
- CH4(l) +2O2(g) →CO2(g) + 2H2O(l)
- CH4(g) +2O2(l) →CO2(s) + 2H2O(l)
- CH4(g) +2O2(g) →CO2(g) + 2H2O(g)
- CH4(l) +2O2(l) →CO2(l) + 2H2O(g)
- CH4(l) +2O2(g) →CO2(g) + 2H2O(g)
(x) Which of these can be reduced when heated with carbon?
- Aluminium
- Calcium carbonate
- Iron (III) oxide
- Magnesium oxide
- Sodium oxide.
2. Match the items in List A with the responses in List B by writing the letter of the correct response beside the item number in the answer booklet provided. Match the items in List A with the responses in List B by writing the letter of the correct
| List A | List B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
3. (a) Define the following terms:
- Neutralization.
- Unsaturated solution.
- Thermal decomposition.
(b) State three main physical properties of water and show the usefulness of each property.
(c) State three industrial application of electrolysis.
4. (a) Copper obtained from copper pyrites (CuFeS2) is impure for electrical wiring and has to be purified by electrolysis.
(i) Name the electrolyte and the electrodes used during electrolysis.
(ii) Write the observations that can be made during the electrolysis.
(b) The following flow diagram shows the stages in the contact process
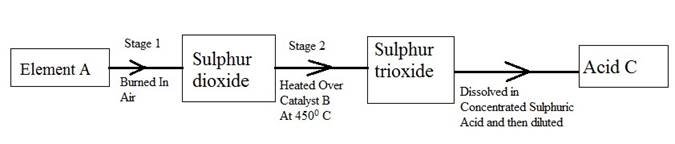
(i) Give the names of element A, catalyst B and an acid C.
(ii) Write a balanced chemical equation for the formation of sulphur trioxide in stage 2
5. (a) Suggest one method of separating each of the following:
(i) Green solution from leaves.
(ii) Alcohol from water.
(b) Elements K, L, M and N have atomic numbers 6, 8, 9 and 20 respectively. Classify each element into its respective period and group.
6. (a) Give one example in each of the following:
(i) Alkali earth metals.
(ii) Noblegases .
(iii) Transition elements.
(b) Write the names of the following processes of changing matter from one state to another.
(i) Gas to liquid.
(ii) Ga s to solid.
(iii) Solid t o gas .
7 (a) Define the following:
( i ) Mole .
( i i ) Molarmass .
(b) 112 dm3 of oxygen gas was collected at s.t.p when a sample of lead nitrate was completely decomposed by heat. Calculate the volume of nitrogen dioxide gas produced.
8. (a) Identify and state the environmental problem caused by the gas which is released from the blast furnace in the extraction of iron from its oxide.
(b) The following equation shows the reaction between hydrogen and iodine gas to form hydrogen iodide gas,H2(g) + I2(g) ↔ 2HI (g) ∆H= -800Kj/mol. Giving a reason, explain what would happen to the position of equilibrium if
(i) temperature is lowered.
(ii) hydrogen iodide gas is pumped into the system.
9. (a) Name two elements which are expected to show similar chemical reaction with magnesium. What is the basis for your choice?
(b) State the main raw material and the process involved in the manufacture of the following products.
- Wood charcoal
- Coke
- Lampblack.
(c) (i) Name the compound which causes temporary hardness of water and the compound which causes permanent hardness of water.
(ii) Write one balanced chemical equation in each case to show how to remove temporary and permanent hardness of water.
10. (a) Suggest one best method for separating each of the following mixtures:
(i) Common salt and water
(ii) Iodine and sand.
(iii) Pieces of iron and sand.
(b) Carbon dioxide can be prepared by adding an acid to calcium carbonate.
(i) Using a named acid, write a balanced chemical equation for the reaction. (ii) Name all the products formed in (b) (i).
11 . ( a ) With the aid of a chemical equation, describe how you would prepare pure solid sodium chloride by the action of an acid and a base.
(b) (i) Why petroleum and coal are non-renewable sources of energy?
(ii) Give three alternatives to non-renewable sources of energy.
12. Three moles of nitrogen gas combine with five moles of hydrogen gas to form ammonium gas by Haber process.
- Which reactant is present in smaller amount?
- Calculate the grams of the reactant left in the container.
- How many moles of NH3 are produced?
- How many litres of NH3 are produced at STP?
SECTION C (15 Marks)
Answer one (1) question in this section.
13.(a) Give three advantages of using chemical equations over word equations.
(b) You are provided with a compound composed of 22.2% zinc, 11.6% sulphur, 22.3% oxygen, and the rest percentage is water of crystallization. Calculate the molecular formula of the compound if its molecular mass is 283.
14. Explain six measures for minimizing the environmental degradation caused by extraction of metals in Tanzania.
FORM THREE CHEMISTRY EXAM SERIES 70
FORM THREE CHEMISTRY EXAM SERIES 70
PRESIDENT'S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ANNUAL EXAMINATION
CHEMISTRY F3- FORM THREE
NAME………………………………………..CLASS……………………………TIME: 3HRS
Instructions:
- This paper consists of sections A, B and C with a total of fourteen (14) questions.
- Answer all questions in sections A and B and one (1) question from section C.
- Cellular phones and any unauthorized materials are not allowed in the examination room.
- Write your Examination Number on every page of your answer booklet(s)
- The following constants may be used;
H = 1, C = 12, N = 14, O = 16, Na = 23, Pb = 207
Al = 27, Cu = 64, Ca = 40, Cl = 35.5, Mg = 24.
Avogadros number = 6.02 x 1023
GMU at stp = 22.4dm3
Faradays constant = 96500C
Standard pressure = 760 mm Hg
Standard temperature = 273K.
SECTION A (15 Marks)
Answer all questions in this section.
- From each of item (i) – (x) choose the correct answer from among the given alternatives and write its letter beside the item number.
- How many moles of sodium carbonate are present in 200cm3 of a 2M solution of the salt?
- 0.4 Mol B. 0.01 Mol C. 0.5 Mol D. 10 Mol
- The chemical properties of an element is determined by;
- Mass number
- Mass spectrometer
- Number of neutrons
- Number and arrangement of electrons
- A solution can be define as a;
- Uniform mixture of two substance
- Heterogenous mixture of solute and solvent
- Homogenous mixture of solute and solvent
- Uniform mixture of two insoluble substance.
- An ion of element Y has 2:8: 8++ as its electronic configuration. The number of proton is the atom of y is;
- 18 B. 16 C. 40 D. 20
- Which of the following chemical can be used to prepare oxygen without applying heat?
- Hydrogen peroxide
- Potassium chlorate
- magnesium oxide
- Zinc carbonate
- Calcium and magnesium are members of group of element in the periodic table called;
- Alkali earth metals
- Alkali metals
- Transition metals
- Amphoteric metals
- In the electrolysis of CUSO4 the weight of copper plate out of the cathode by a current of 0.70 Amps flowing for 10 minutes is;
- 0.139gm B. 10.48gm C. 14.48gm D. 1.148gm
- In the reaction;
2FeCl2 + Cl2 ![]() 2FeCl3
2FeCl3
Chlorine may be regarded as;
- A reducing agent
- An oxidizing agent
- A catalyst
- Halogens
- When 0.125 Faraday of electrolysis are passed through a copper (II) Sulphate soln. The mass of copper deposited will be;
- 4g B. 8g C. 64g D. 32g
- The percentage of water of crystallization in MgSO4.7H20 is;
- 53.4% B. 51.2% C. 49.2% D. 47.3%
- Match the item in list A with their responses in List B by writing the letter of the correct response against the item number;
| LIST A | LIST B |
|
|
SECTION B (70 Marks)
Answer all questions in this section.
- (a) Differentiate between;
- Atomic number and Atomic mass
- Distillation and sublimation
(b) Define the following terms;
- Molar solution
- Ion
- Molar mass
- Neutralization
- (a) How many electrons will be needed to discharge 0.135g Aluminium?
(b) A solution of acetic acid (CH3COOH) was made by dissolving 3.00g of the acid in 0.5
dm3 of solution. Upon titration 12.00cm3 of this solution required 25.00cm3 of
sodium hydrogen carbonate for complete neutralization. Calculate the molarity of the
bicarbonate (NaHCO3).
The ratio of the reacting moles is 1:1.
- (a) Give the name of substance or apparatus which fits the following descriptions;
- It is used for measuring fixed volume of liquid
- It is used for holding and keeping apparatus up right.
- It is used as a drying agent.
(b)Write down three differences between potassium ions and potassium atom.
- Carefully study the electronic configuration of the following element;
- : 2 : 4 B. : 2 : 7 C. : 2 : 8 D. : 2 : 8 : 2
E. 2 : 8 : 8 : 2F = 2 : 8 : 6
(a)Which of the above elements would you expect to be;
- Metallic
- Non metallic
- Inert gases
(b)What would you expect to be the formula of the compound formed in each of the following pairs;
- A with B
- B with E
- D with F
(c)Deduce the period and group in the periodic table of;
(i)E(ii) F
(d)Write down a balanced chemical equation showing what would happen if an oxide of element E was treated with dilute hydrochloric acid.
- (a) Differentiate between water treatment and water purification.
(b) Mention three methods of purifying domestic water.
(c) What causes the following;
i. Temporary hardness in water
ii. Permanent hardness in water
(d) i. With the aid of chemical equations explain why it is possible to soften temporary
hardness by boiling.
ii. Mention one advantages of using hard water.
- (a) State Faraday laws of electrolysis.
(b) An element Z has a relative atomic mass of 88. When a current of 0.5 amperes was
passed through fused chloride of Z for 32 minutes and 10 seconds 0.44 of Z were
deposited at the cathode;
- Calculate the number of Faradays needed to liberate one mole of Z.
- Write the formula of the Z ions
- Write the formula of hydroxide of Z.
- (a) What is the meaning of the following terms;
- Exothermic reactions
- Endothermic reactions
(b) Draw the energy – level diagram for exothermic reactions and Endothermic reactions.
(c) Name four (4) characteristics of good fuel.
- 20cm3 of solution containing 7g/dm3 of metal hydroxide XOH, were exactly neutralized by 25cm3 of 25 cm3 of 0.10M hydrochloric acid.
(a)Write a balanced chemical equation for neutralization of metal hydroxide XOH.
(b) Calculate the concentration of metal XOH in mole per dm3.
(c)i. Calculate molar mass of XOH
ii. Identify element X.
- (a) i. List down three (3) factors affecting the selection of ion discharge at the electrode.
ii. Define the term electrolyte.
(b) A bluish copper sulphate aqueous solution was electrolysed by using copper
electrodes.
- Write ionic chemical equations for the reactions which occurred at the cathode and anode.
- Explain what will happen to blue colour of copper sulphate solution as electrolysis continues.
(a) Define and point of titration
(b) What colour change indicate end point in the titration of a base against an acid on using (i) Methyl orange?(ii) Phenolphthalein?
(c) A teacher of chemistry provided his F3 students with the following:
AA – A solution of 0.12 0M HCl
BB – A solution of hydrated sodium carbonate, Na2CO3 containing 14.30g/dm3. On titration by using a 20cm3 pipette one of the best student obtained the following readings from a standard burette
| Burette readings | Pilot | 1 | 2 | 3 | 4 |
| Final volume (cm3) | 18.00 | 35.90 | 27.90 | 45.50 | 27.60 |
| Initial volume (cm3) | 0.00 | 18.00 | 10.20 | 27.90 | 10.00 |
| Volumeused (cm3) |
(i)Suggest the colour change at the end point
(ii)Suggest the indicator used by the student in the titration
(iii)Complete the table above
(iv)Calculate the average volume used for complete neutralization of the base by acid
(v)Find the value of X in the formula Na2CO3X H2O
12.(a) State three main physical properties of water and show the usefulness of each property.
(b)State three industrial application of electrolysis.
SECTION C (15 Marks)
Answer one (1) question from this section.
12. Explain how to handle chemicals having the warning signs of flammable, corrosive, harmful, explosive and toxic in the laboratory.
13. Explain six measures for minimizing the environmental degradation caused by extraction of metals in Tanzania.
FORM THREE CHEMISTRY EXAM SERIES 35
FORM THREE CHEMISTRY EXAM SERIES 35
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256