THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
132/1 CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 Hours Year: 2010
Instructions
1. This paper consists of sections A and B with a total of ten (10) questions.
2. Answer all questions in section A and two (2) questions from section B.
3. Each question carries ten (10) marks in section A and fifteen (15) marks in section B.
4. Mathematical tables and non-programmable calculators may be used.
5. Cellular phones and any unauthorised materials are not allowed in the examination room.
6. Write your Examination Number on every page of your answer booklet(s).
7.For calculations, you may use the following
Rydberg constant, RH=1.09678x 107m-1
Gas constant, R = 8.31JmoI-1K-Ior 0.0082 atm mol-1K-Idm3
GMVat s.t.p.22.4dm3
1 litre = 1 dm3=1000cm3
At STP: Temperature = 273K, Pressure = 760mmHg
Planck constant, h=6.63x 10-34Js
Velocity of light, C = 3.0 x 108m/s
Atomic masses H = 1, C = 12, N = 14, O-16, Na-23
1.(a) Define the following:
- Isotopes
- Atomic number
- Mass number
- Radioactivity
View Ans
(b)Explain why818Z and816Z atoms have identical chemical properties but90134X and94134X have different chemical properties.
View Ans
(c)X is a radioactive element which undergoes transition as follows:

If the atomic number of X is 17 and its mass number is 37. What are the atomic numbers and mass numbers of isotopes Y, Z and W?
View Ans
(d)Describe three (3) applications of radioactivity.
View Ans
2. (a) Electronic configuration of silver violates Aufbau's building principle. Justify this statement and explain briefly the observed violation.
View Ans
(b)(i) Define the term quantum numbers.
(ii)What are the four (4) properties described by quantum numbers?
View Ans
(c)Why do ionization energies increase from left to right across the periodic table?
View Ans
3.(a) Define the following giving one example in each case:
(i) Hydrogen bond
(ii) Coordinate bond (iii) Polar covalent bond (iv) Electrovalent bond.
View Ans
(b) (i) Describe the conditions necessary for the formation of hydrogen bond. How does the described bond differ from other intermolecular forces?
(ii)With the help of diagrams, show the type of hybridization, geometry and bond angle found in methane, ethene and ethylene molecules.
(iii)By using diagrams, explain why NH3 and CH4 have tetrahedral geometrical structures but the bond angle in CH4 which is 109.50is greater than in NH
View Ans
4.(a ) State the following:
(i)The equilibrium law
(ii)LeChaterlier's principle.
View Ans
(b)Explain briefly the following: (i) Dynamic equilibrium (ii) Reaction quotient.
View Ans
(c)Dinitrogen tetraoxide in its liquid state was used as one of the fuels on the lunar Lander expeditions for a NASA space vessel. In the gas phase it decomposes to gaseous nitrogen dioxide as shown in the following equation:
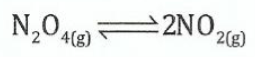
N2O4was allowed to reach equilibrium at 4000C where Ksp= 0.133atm. At equilibrium the pressure of N2O4(g) was found to be 2.71atm.
(i)Write the equilibrium expression in terms of the concentration.
(ii)Write the equilibrium expression in terms of the partial pressures. (iii) Calculate the equilibrium pressure of NO2(g)
View Ans
5.(a) Define the following terms:
(i)Colligative properties
(ii)Ideal solution
View Ans
(b)List the following in the order of increasing boiling points: 0.03M hexane, 0.03M Potassium chloride and 0.03M acetic acid.
Potassium chloride and 0.03M acetic acid.
View Ans
(c)(i) The melting point of camphor is 176.50C. When0.125g of sulphur is finely ground and mixed with 3.62g of camphor, the resultant mixture melts at 171.40C. What is the molecular weight of sulphur in camphor?
(ii)Water and ethanol form an azeotropic mixture of composition 95.6% ethanol which boils at 78.150C. Sketch a well labelled temperature composition curve for the water-ethanol mixture. Explain whether it is possible to obtain pure ethanol from a water-ethanol mixture.
View Ans
6.(a) Give a brief explanation of the following terms:
(ii)Molarity
(iii)Avogadro's constant
(iv) A molar solution
View Ans
(b)One eighth of a mole of a certain hydrated salt contains 112g of water. Calculate the number of molecules of water of crystallization of the salt.
View Ans
(c)The atomic radius of sodium is cm and the molar volume of sodium is 23.68cm3. If 68.52% of this volume is the actual volume occupied by sodium atom, calculate the Avogadro's constant. (Volume of one sodium atom = ?x3).
?x3).
View Ans
7.(a) The empirical formula of nicotine, a poisonous compound found in tobacco is C5H7N. Its molecular weight is 162. What is the molecular formula of the compound?
View Ans
(b)How many grams of oxygen are required to burn 57.0g of octane?
View Ans
(c)What is the arrangement of electrons in62}Co which is used in cancer therapy?
View Ans
(d)One mechanism for the removal of excess heat generated by metabolic processes in the body is evaporation of the water in sweat. In a hot, dry climate as much as 1.5 litres per day may be lost by one person. Calculate the amount of heat required to evaporate this water at T = 460C. (AH vap = 43.02kJmo F1at 460C)
View Ans
8.In what ways does the chemistry of hydrogen resemble that of:
(i) alkali metals(ii) halogens?
View Ans
(b)Explain the meaning of the following terms:
(i)Lone pair electrons (ii) Electronegativity(iii) Electron affinity.
View Ans
(c)Describe two industrial methods for the preparation of hydrogen.
View Ans
9.State the allotropic properties of carbon.
View Ans
(b)Account for the following:
(i)Ba and Mg are group 2 elements but Mg(NO3)2does not impart colour to the Bunsen burner flame while Ba(NO3)2does
(ii)K+and Ca2+have the same number of electrons but the size ofK+is larger than Ca2+
(iii) Be is in period two and group two while Al is in period three and group three BUT the properties of their of their compounds resemble in most aspects
Be is in period two and group two while Al is in period three and group three BUT the properties of their of their compounds resemble in most aspects
View Ans
(c) Explain why the acidity of the hydrides of group seven elements increase down the group.
View Ans
10.(a) Explain briefly the following terms:
(i)Indicator
(ii)Titrant
(iii)End - Point.
View Ans
(b) Commercially available concentrated hydrochloric acid is an aqueous solution containing 38% HCI acid by mass and has a specific gravity of 1.18g/cm3.
(i)Calculate its molarity.
(ii)How many cm3of concentrated hydrochloric acid are required to make 25cm3of 0.01M HCI solution?
View Ans
11.Write the IUPAC names for the following compounds:
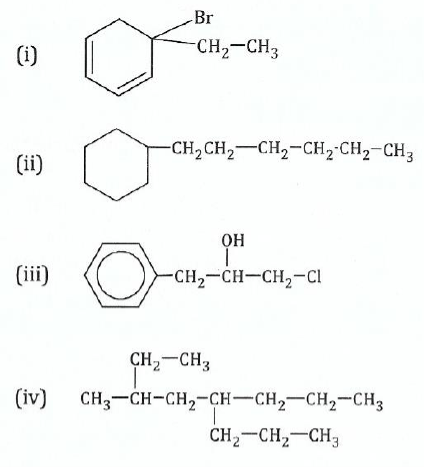
View Ans
(b)Write the structural formulae of the following compounds:
(i)Propan-l-ol
(ii)4-methylcyclohex-l-ene
(iii)I-isopropyl-methylcyclohexane
(iv)4-methylpent-2-yne.
View Ans
(c)Write the equations for suitable laboratory methods of preparing the following alkanes starting from one organic compound that has less than four carbon atoms only.
(i)2,3-dimethyIbutane. (ii) Pentane.
View Ans
12.(i) Write the structural isomers of molecular formula C4H9Cl.
(ii) Explain by using chemical equations the chemical test which can be used to distinguish the isomers in 12 (a) (i).
View Ans
(b)Write the equations for the following reactions:
(i)Benzene and chloromethylbenzene (benzyl chloride), in the presence of AIC13.
(ii)I-chloromethyI-4-nitrobenzene and benzene in the presence of AICI3
View Ans
(c) Name the following compounds:
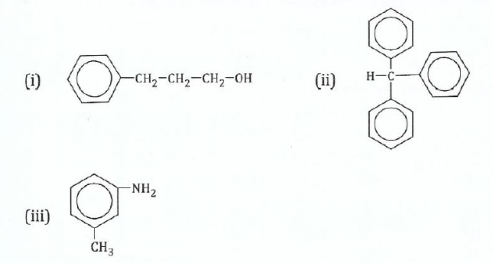
View Ans
13.Write chemical equations showing how you would prepare the following compounds:
(i)Butan-2-ol from 2-iodobutane
(ii)Propane from I-bromopropane
(iii)I-iodobutane from I-bromobutane
(iv)Methoxymethane from chloromethane
(v)Butane from ethane
(vi)Methylbenzene from acetylene
View Ans
(b)Suggest suitable tests to distinguish the following compounds:
(i) But-2-yne and butane
(ii) Cyclobutane and pent-2-ene
(iii) Methanol and ethanol
(iv) 2-butyne and I-butyne.
View Ans
14.A student gave the following names for various alkanes:
(i) 2, 4- dimethylbutane
(ii) 2, 5-diethyl-4-methylheptane
(iii) 2-propylpentane
(iv) l, l-diethyl-l,l-dipropylmethane.
The names indicate the structural formulae of the alkanes but do not strictly follow the IUPAC rules of nomenclature.
Draw the structural formulae suggested by the incorrect names and give the correct IUPAC name for each compound.
(b) Write equations to show how you could prepare the following compounds:
(i) Ethyl ethanoate from chloroethane
(ii) Ethanoic acid from ethane.
(iii) 1,2-dichloroethane from ethanol.
(c) By considering the typical functional group reactions, suggest the products of the
reactions between compound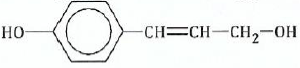
and the following reagents:
(i)Dilute alkaline potassium permanganate.
(ii)Acidified potassium permanganate. (iii) Ethanoyl chloride.
View Ans
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256